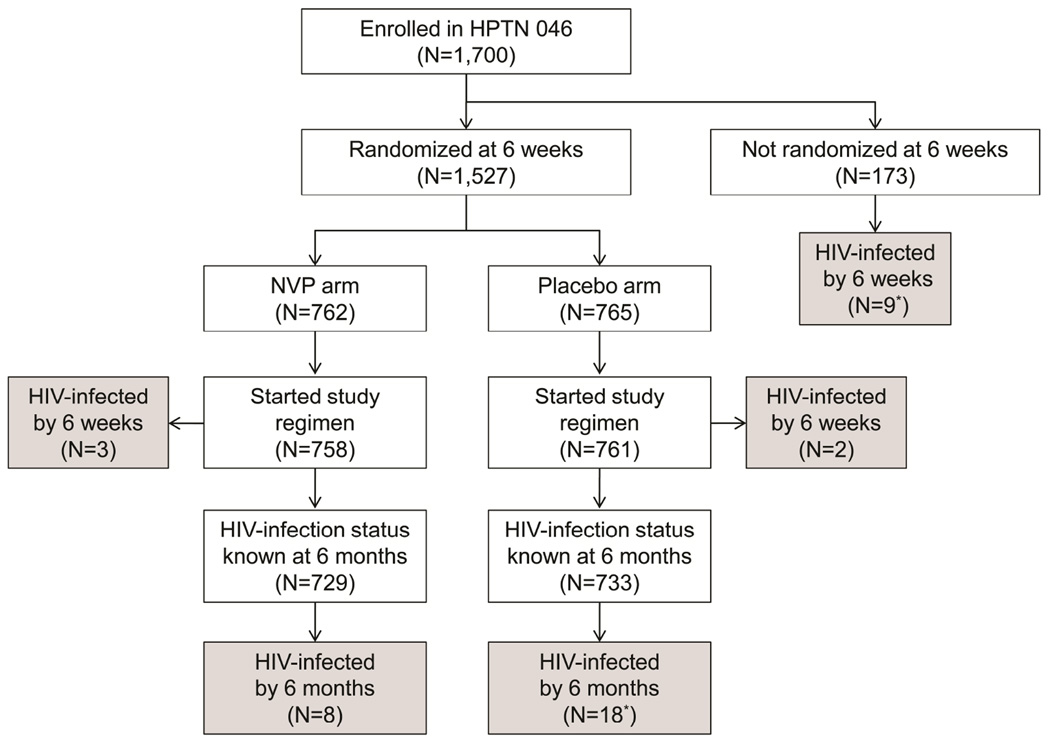
Figure 1.
HIV genotyping results obtained for maternal plasma, breast milk, and infant plasma samples from eight mother-infant pairs where the mother started highly active antiretroviral therapy (HAART) by 6 months postpartum; all available samples were tested. Samples were collected at 6 weeks, and at 3 and 6 months postpartum. Shaded boxes indicate the study visits the mother was on HAART. HIV genotyping was not performed for maternal samples that had <400 copies/ml (c/ml) HIV RNA (VL<400c/ml). (a) A nested polymerase chain reaction (PCR) procedure was required for amplification. (b) HIV genotyping results for this infant did not include amino acids 8 to 32 in HIV reverse transcriptase. No major non-nucleoside reverse transcriptase inhibitor (NNRTI) or nucleoside / nucleotide reverse transcriptase inhibitor (NRTI) resistance mutations occur in that region of the reverse transcriptase protein.