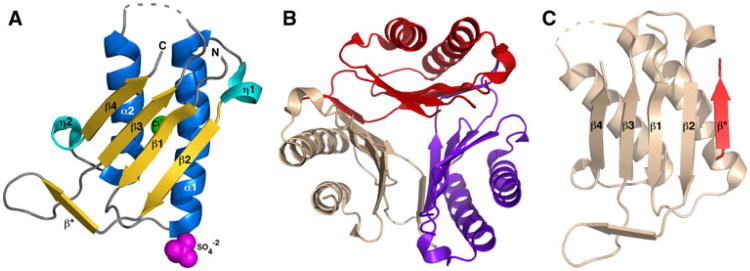
Fig. 1.
Crystal structure of the macrophage migratory inhibitory factor (MIF) from Giardia lamblia. a Ribbon representation of a single molecule of Gl-MIF in the asymmetric unit of the crystal. The elements of secondary structure are labelled and coloured marine (α-helix), cyan (310-helix (η)), and gold (β-strand). The sulfate and chloride ions are shown as spheres coloured magenta and green, respectively. The dashed grey line represents a region of the protein with no interpretable electron density suggesting the region is disordered. b The biological relevant Gl-MIF trimer viewed down the three-fold axis with each protein subunit coloured red, wheat, and purple. c Example of the intermolecular β-strand formation between two protein units in the trimer