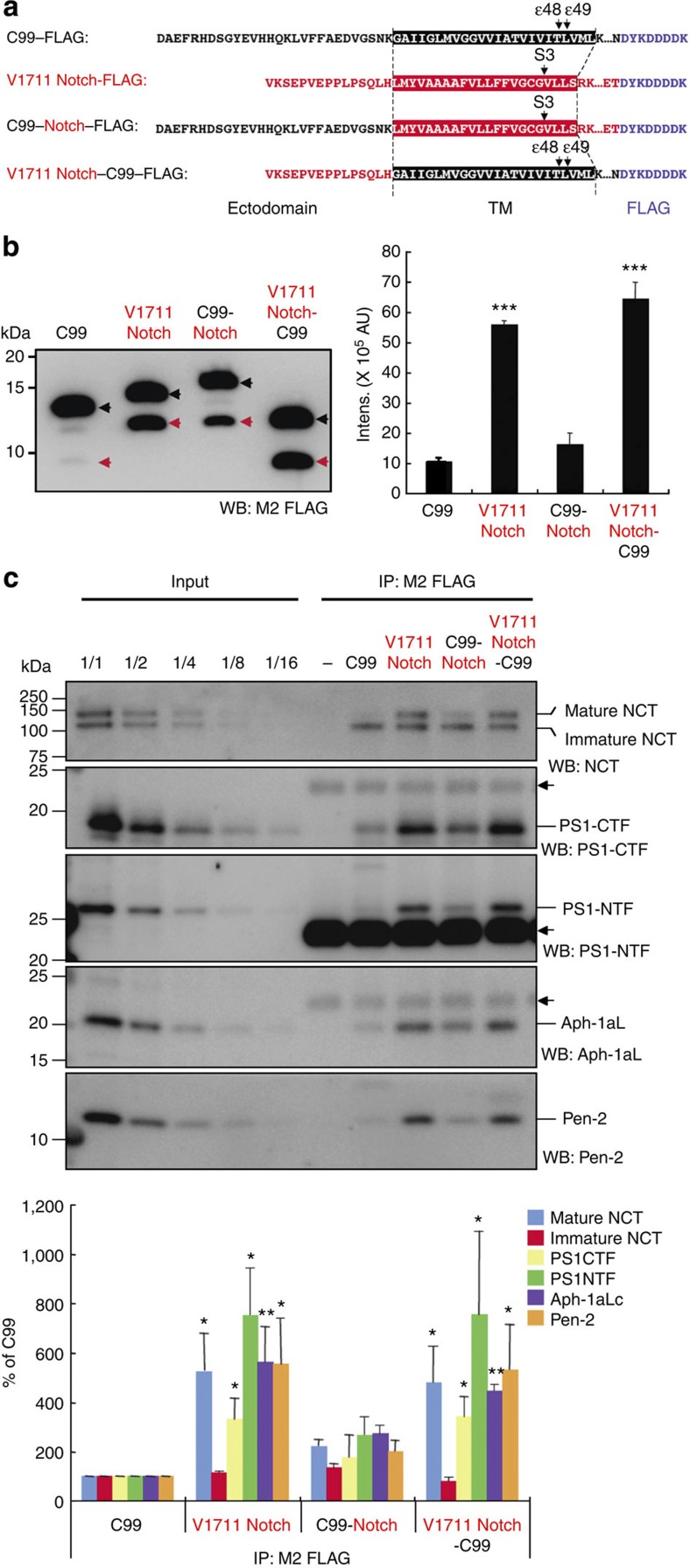
Figure 4. Ectodomain swapping of C99 and V1711 Notch substrates.
Diagram showing C99, V1711 Notch and their chimeric substrates (a). Black and red indicate C99 and V1711 Notch-derived sequences, respectively. The boxed sequence indicates the transmembrane region of the substrate. Arrows indicate cleavage sites on each substrate. Chimeric substrates were incubated with CHAPSO-solubilized γ-secretase fractions and western blotting was used to visualize and quantify the intracellular domain released from the substrate (b). Substrates containing the ectodomain of V1711 Notch were efficiently cleaved by γ-secretase. Black arrowhead, substrate; red arrowhead, intracellular domain. Intensity of each band of intracellular domain was quantified using LAS-4000 luminescent image analyser and plotted in arbitrary unit. Data are expressed as means±s.d. of three independent experiments. ***P<0.0005 (analysis of variance (ANOVA), Scheffe’s post hoc test compared with C99). Substrates were immobilized using anti-FLAG M2 magnetic beads and mixed with CHAPSO-solubilized γ-secretase fractions (c). After sufficient washing of co-immunoprecipitates, western blotting was used to visualize and quantify γ-secretase components. Substrates containing the ectodomain of V1711 Notch (V1711 Notch-FLAG and V1711 Notch-C99-FLAG) had increased interaction with γ-secretase components. By contrast, substrates containing C99 ectodomain decreased interaction with γ-secretase components. Interestingly, immature nicastrin bound to any substrate equally. Arrows indicate the position of immunoglobulin G used for co-immunoprecipitation. Data are expressed as means±s.d. of three independent experiments. *P<0.05, **P<0.005 (ANOVA, Scheffe’s post hoc test compared with C99).