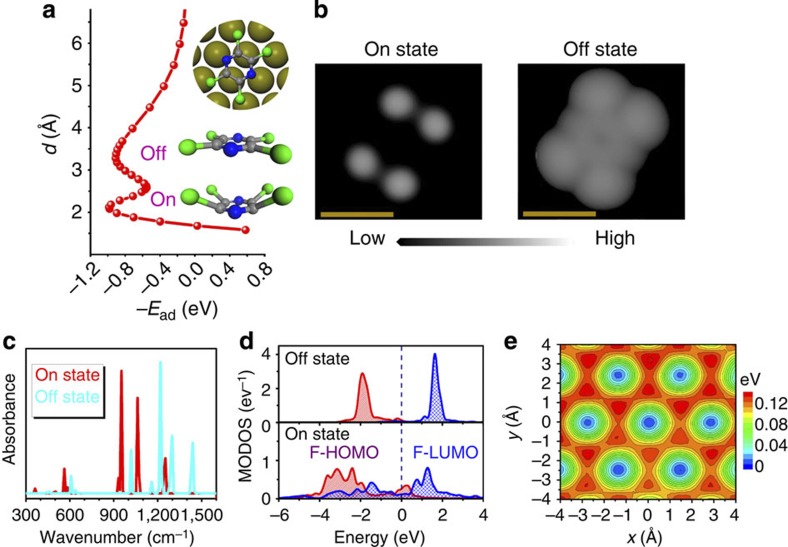
Figure 1. Bistable behaviour of tetrachloropyrazine on Pt.
(a) Adsorption energy −Ead (x axis) as a function of the adsorption height d (y axis) for tetrachloropyrazine (C4N2Cl4) adsorbed on the Pt(111) surface at the most stable bridge site, with an angle of 30° between the C–N and Pt–Pt bonds (the so-called ‘bridge-30°’ adsorption site; see inset plot). Two stable ground states at different d are clearly represented in the binding curve, with almost equal stability and a moderate activation barrier between the two states. (b) Constant-current scanning tunnelling microscopy (STM) simulation using the Tersoff–Hamann approximation for C4N2Cl4 on the Pt(111) surface at a positive bias voltage of 40 mV. The difference between the two STM images is mainly attributed to the planar (off state) and distorted (on state) structures of the adsorbate. (c) Calculated infrared spectra of C4N2Cl4/Pt(111). Formation of covalent bonds between the adsorbate and the substrate results in a red-shift of the vibrational spectra. (d) Molecular orbital density of states (MODOS) of C4N2Cl4 on Pt(111). The former highest occupied molecular orbital (F-HOMO) and former lowest unoccupied molecular orbital (F-LUMO) remain unperturbed in the off state, whereas they strongly overlap in the on state. (e) Contour plot of the potential-energy surface for the C4N2Cl4 molecule on the Pt(111) surface in the off state (with d=3.14 Å). (b) Scale bar, 0.5 nm. For more details on the methodology refer to the Methods section.