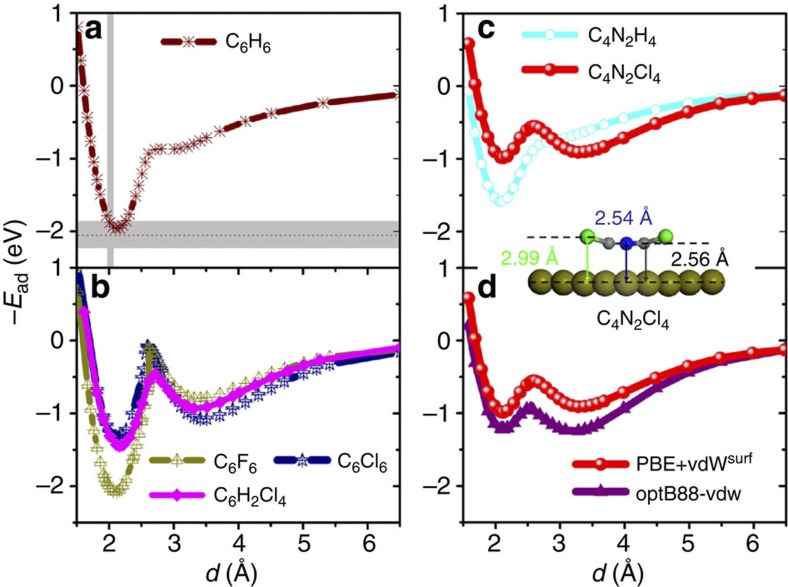
Figure 2. Generality of the bistable behaviour for benzene derivatives.
(a) Adsorption energy for benzene (C6H6) adsorbed on the Pt(111) surface at the most stable bridge-30° site as a function of distance d from the surface. The grey intervals indicate the experimental binding distance based on a low-energy electron diffraction (LEED) analysis45 and adsorption energies determined from microcalorimetry measurements36. (b) The binding energy curves for hexafluorobenzene (C6F6), hexachlorobenzene (C6Cl6) and 1,2,4,5-tetrachlorobenzene (C6H2Cl4) on Pt(111). (c) Two additional curves for tetrachloropyrazine (C4N2Cl4) and pyrazine (C4N2H4) on Pt(111). The inset shows the structure of the transition state for the C4N2Cl4 molecule. (d) Comparison of the binding energy curves from the PBE+vdWsurf and optB88-vdW methods for the C4N2Cl4/Pt(111) system. For each structure, we fixed the z coordinates of the carbon backbone and the Pt atoms in the bottom four layers of the six-layer surface model. The adsorption height d corresponds to the perpendicular C–Pt distance, which is evaluated relative to the position of the unrelaxed top metal layer.