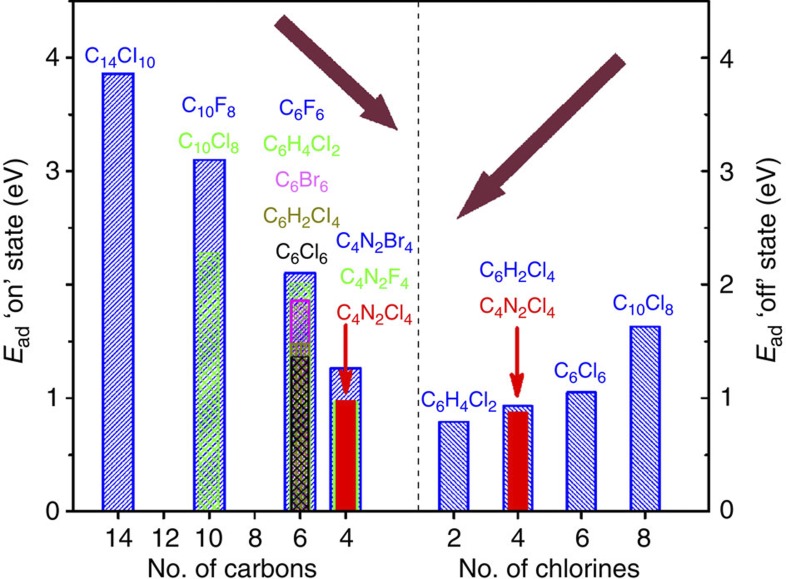
Figure 3. Summary of adsorption energies of the two states.
Adsorption energy Ead (eV) in the on (left panel) and off (right panel) states, respectively, as a function of the number of carbon and chlorine atoms in each molecule adsorbed on the Pt(111) surface. The adsorption energy in the on state decreases with decreasing number of carbon atoms, whereas the adsorption energy in the off state increases with an increase in chlorine atoms. The tetrachloropyrazine (C4N2Cl4) molecule has roughly the same stability in the two states (indicated by red arrows).