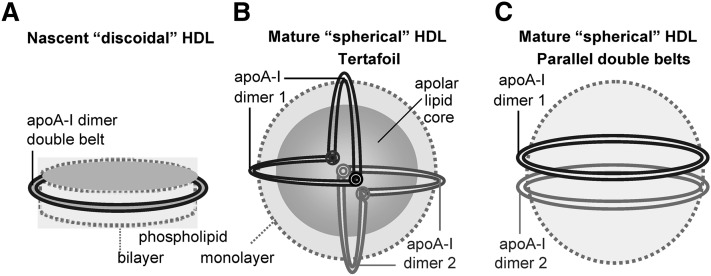
Fig. 1.
Cartoon representation of apoA-I conformations on various HDL. (A) Nascent “discoidal” HDL contains two copies of apoA-I encircling the phospholipid bilayer in an antiparallel, double-belt conformation (4, 5). This conformation is also shown as a ribbon diagram in supplementary Fig. II. (B) Mature spherical HDL contains a core of apolar lipids (mainly cholesteryl esters and triacylglycerides) surrounded by the protein-containing phospholipid monolayer. Large HDL contains two molecular dimers of apoA-I that are proposed to form a tetrafoil on the particle surface (7, 8). We proposed that each double belt in this tetrafoil is bent around two flexible Gly-containing hinges (circles) (9). The two dimers are in different shades of gray. (C) If the double-belt bending around the flexible hinges is impaired, the two apoA-I double belts are expected to form two parallel rings on the HDL surface. We propose that this parallel arrangement, which was first proposed in the cross-linking studies of rHDL(A-IM/A-IM) (10), may be a general property of apoA-IM and, possibly, apoA-IP homodimers (Fig. 8 and supplementary Fig. V). Importantly, these and all other models presented in this work illustrate an overall protein arrangement but not the small local conformational adjustments that are probably involved in the double-belt formation by the mutant proteins.