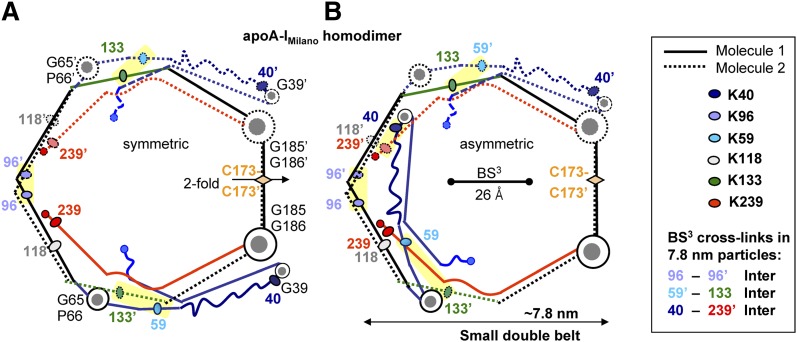
Fig. 3.
Chemical cross-links identified in ∼7.8 nm rHDL(A-IM/A-IM) (10) mapped on the proposed model of the small A-IM/A-IM double belt. (A) Symmetric double-belt contains two apoA-IM molecules in a conformation depicted in Fig. 2D. The two-fold symmetry axis passes through theC173-C173′ disulfide. (B) In Molecule 1 of the asymmetric double belt, segment 1–65 (in blue) is folded back around the G65-P66 hinge. Line and color coding is as in Fig. 2. The major intermolecular cross-links are highlighted. Cross-linked Lys are shown by ovals; residue numbers are color-coded. These cross-links are facilitated by the lysine locations in the “left” faces of the amphipathic α-helices, which are proximal in the double belt (5, 38). Thus, K96 is located in the middle of the “left” helical face that is facing its symmetry mate in the double belt, thereby facilitating the K96-K96′ cross-link. Similarly, K133 is located in the “left” helical face, facilitating its cross-link with L59 that is located in the “left” face of the short helix observed in the crystal structure (9). Also, K239 is located in the “left” helical face, which is expected to facilitate its cross-linking to K40 located in the turn between the helices in the crystal structure (53). Bar size shows the maximal Cα-Cα distance of 26 Å that can be cross-linked by BS3 used by Bhat and colleagues (10).