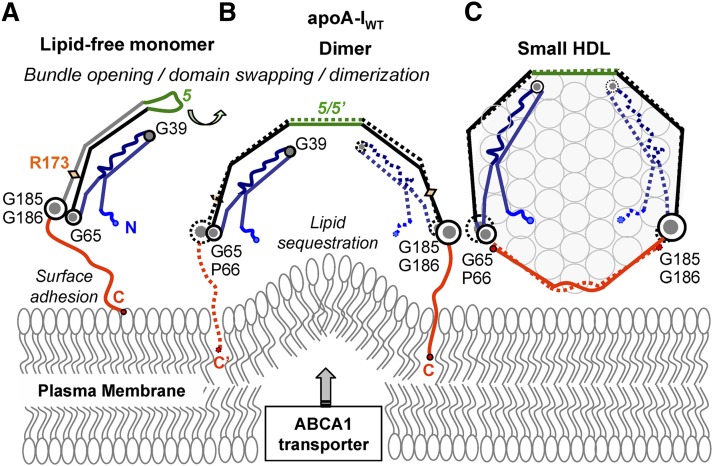
Fig. 8.
Proposed structural changes in the apoA-IWT protein and lipid during the initial rate-limiting steps of reverse cholesterol transport. Lipid-poor/free monomeric apoA-IWT forms a globular four-segment bundle as previously described (53, 55) (A). Upon adhesion to the lipid surface via the flexible hydrophobic C-terminal segment 185–243 (in red), apoA-IWT dimerizes via the domain swapping around repeat 5 (in green) (53), which is followed by lipid sequestration (9) (B). This process generates small nascent HDL containing one copy of A-IWT–A-IWT double belt wrapped around the perimeter of a phospholipid bilayer (C). In C, nascent discoidal HDL are shown face-up, and phospholipid head groups are show in circles. Lipid efflux from the plasma membrane to apoA-I is mediated by the lipid transporter ABCA1 that forms protrusions on the plasma membrane and thereby increases lipid availability to apoA-I (Refs. 13–15 and references therein).