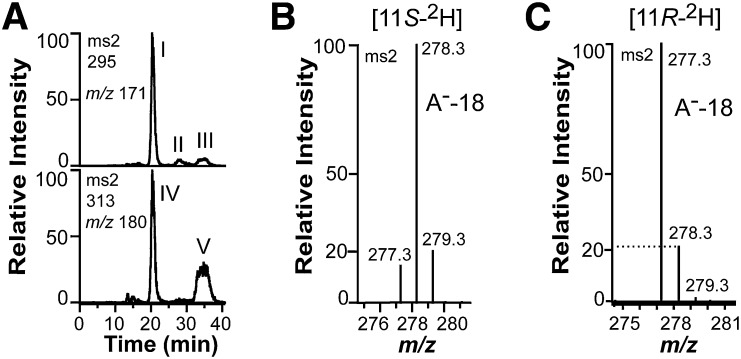
Fig. 3.
Oxidation of 18:2n-6, [11S-2H]18:2n-6, and [11,11-2H2]18:2n-6 by recombinant 9S-DOX-AOS (FOXB_01332). A: Steric analysis by CP-HPLC-MS/MS of the main hydroperoxide formed from 18:2n-6 by 9S-DOX-AOS after reduction to an alcohol (top trace). Small amounts of racemic [13C18]9-HODE were added to facilitate the analysis (bottom trace). The main biological product after reduction of hydroperoxides to alcohols, peak I, coeluted with the 9S stereoisomer of [13C18]9-HODE (peak IV) and not with [13C18]9R-HODE (peak V). Small amounts of 9R-HODE (peak III) and trans-trans 9-HODE (peak II) were also detected (top trace). B: Partial MS/MS spectrum of 9-HODE from oxidation of [11S-2H]18:2n-6 (95% 2H) by recombinant FOXB_01332. The deuterium label was retained as judged from the prominent signal at m/z 278 [296-18; loss of water from the carboxylate anion (A−)]. C: Partial MS/MS spectrum of 9-HODE from oxidation of [11R-2H]18:2n-6 (25% 2H) by the recombinant enzyme. The deuterium label was not retained as judged from the signals at m/z 277 and 278 [loss of water from the carboxylate anions (A−)]. The intensity of the latter ion was ∼20% of the intensity of m/z 277, and this is in agreement with the natural abundance of the 13C isotope (1.1% 13C at each of the 18 positions).