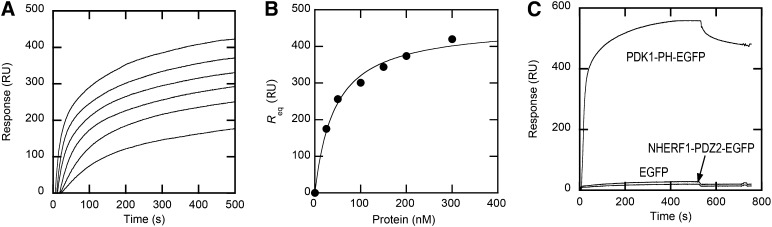
Fig. 2.
Determination of Kd for PDK1-PH-EGFP membrane binding by SPR analysis. A: Equilibrium sensorgrams for PDK1-PH-EGFP interacting with POPC/POPS/PtdIns(3,4,5)P3 (77:20:3) vesicles. The protein concentration was 25, 50, 100, 150, 200, and 300 nM from bottom to top. B: Binding isotherms of PDK1-PH-EGFP with POPC/POPS/PtdIns(3,4,5)P3 (77:20:3) vesicles. The binding isotherm was fit by nonlinear least-squares analysis using the equation: Req = Rmax/(1 + Kd/[P]o) where [P]o, Req, and Rmax are the total protein concentration, the near-equilibrium resonance unit (RU) value for each P0, and the maximal Req value, respectively. Rmax (460 ± 20) RU and Kd (44 ± 15 nM) values were used to construct the theoretical curve (solid line). C: Kinetic sensorgrams for PDK1-PH-EGFP, NHERF1-PDZ2-EGFP, and EGFP (500 nM each) interacting with POPC/POPS/PtdIns(3,4,5)P3 (77:20:3) vesicles coated onto the L1 chip.