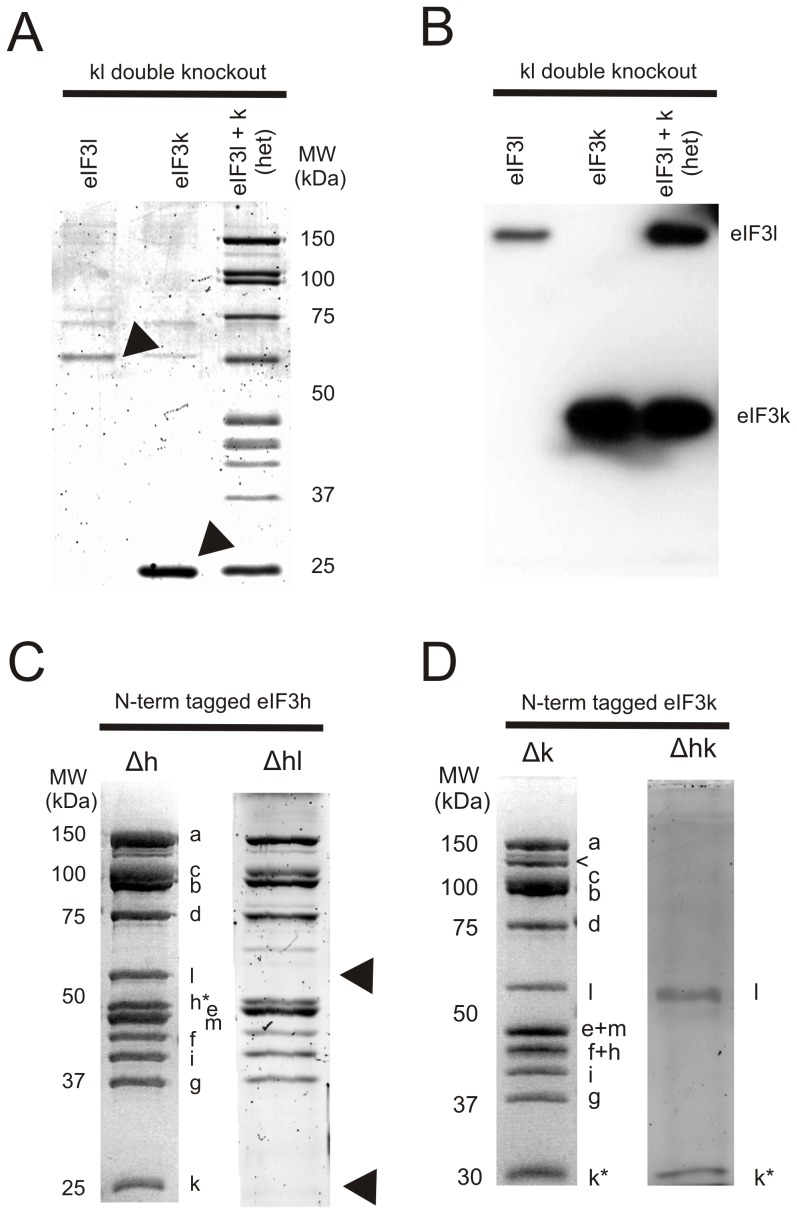
Figure 4. Affinity purification of eIF3 knock-out strains reveal subunit interdependence during eIF3 assembly.
(A) Anti-FLAG affinity purifications from N. crassa extracts using N-terminally tagged eIF3l or eIF3k in the ΔeIF3kl double knock-out background (lanes 1 and 2). Affinity purification from N. crassa extract obtained from a heterokaryon strain (lane 3) made from strains used in lanes 1 and 2. N-terminally tagged k and l subunits expressed from the his-3 locus are indicated above each lane. All strains are ΔeIF3kl double knock-outs. Contaminant bands in lanes 1 and 2 were identified as phenylalanyl tRNA synthetase beta chain (73 kDa) and tRNA ligase (58 kDa). (B) Anti-FLAG Western blot of the affinity purifications from the gel in (A). Each lane has the exact amount of total protein loaded in (A). Anti-FLAG affinity purifications from N. crassa extracts using N-terminally tagged eIF3h in ΔeIF3h or ΔeIF3hl strains (C) or N-terminally tagged eIF3k in ΔeIF3k or ΔeIF3hk strains (D). The tagged h or k subunits are indicated with asterisks. Arrows indicate the missing k and l subunits in the ΔeIF3hl strain (C) or a degradation product of eIF3a (D). All gels in panels (A), (C) and (D) are stained with Coomassie blue.