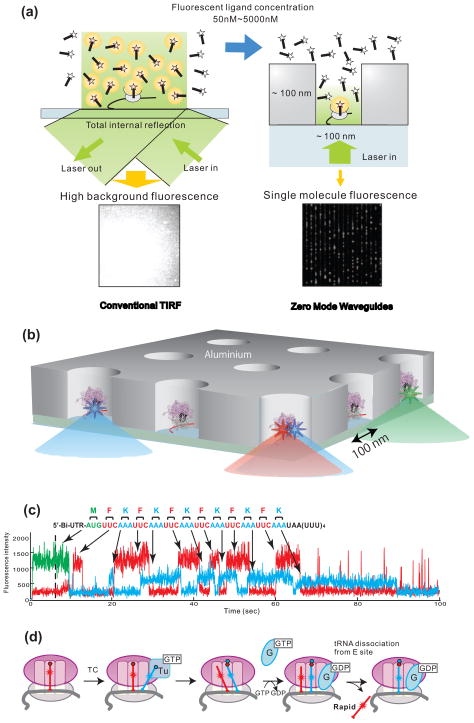
Figure 4. Visualization of single molecule translation in ZMWs.
(a) Comparison between conventional TIRF and use of zero-mode waveguides (ZMWs). At concentrations of fluorescently labeled ligands higher than ~50 nM, spots are not distinguishable due to high background fluorescence (left). ZMWs overcome this limitation (right) by decreasing illumination volume by three to four orders of magnitude compared to TIR excitation to an order of zeptoliters (10−21 L). This allows fluorescence detection in the μM range. (b) Schematic illustration of ZMW construction. Ribosomal complexes are specifically immobilized in the bottom of derivatized ZMWs using biotinylated mRNAs. Initial ribosome complex immobilized to the bottom surface of a ZMW contains Cy3-labeled fMet-tRNAfMet
(c) Fluorescence is excited by illumination at 488, 532 and 642 nm, and Cy2, Cy3 and Cy5 fluorescence are simultaneously detected. Heteropolymeric mRNAs encoding 13 amino acids were used: M(FK)6. Translation was observed in the presence of Phe-(Cy5)tRNAPhe, Lys-(Cy2)tRNALys complexed with EF-Tu and EF-G as a series of fluorescent pulses that mirror the mRNA sequence. A long Cy2 pulse is observed upon arrival of the ribosome at the stop codon. (d) Model for tRNA transit through the ribosome during translation. Blue-labeled tRNA arrives in the A-site, resulting in a 2-tRNA state with a red P-site-bound tRNA. This state forms an A/P hybrid state after peptide bond formation. Subsequent translocation of the 2 tRNA-mRNA complexes to the E and P sites is followed by rapid dissociation of red E-site tRNA. Reproduced with permission from Uemura et al., 2010.