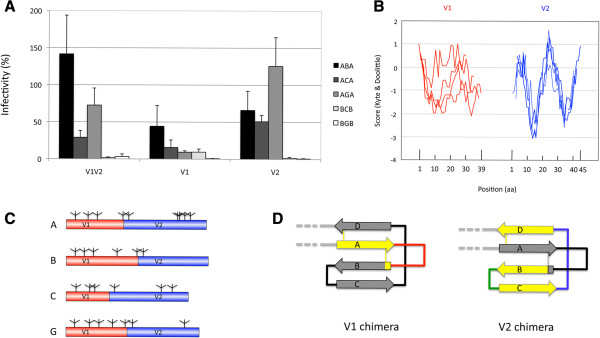
Figure 4.
Implication of variable domains V1 and V2 in the loss of functionality of the chimeras.Panel A. Functionality of V1 and V2 chimeras. The values for the V1V2 chimeras (from Figure 2A) are given as a reference for comparison with the V1 and V2 chimeras. The values are the average of 3 to 6 independent experiments. In all cases, the values are given relative to those observed for the corresponding wild-type receiver protein. Panel B. Hydrophobicity profile of the variable regions V1 and V2. The profile of the four sequences most intensively studied here (isolates A, B, C, and G) is given for V1 in red and for V2 in blue. The position in amino acids is given on the x axis, with numbering starting from the first amino acid (N-terminal) after the cysteine residue that marks the border of the C1-V1 transition for V1 and the V1-V2 transition for V2. Panel C. Potential N-Glycosylation sites of the variable regions V1 and V2 for each subtype used. The locations of the N-glycosylation sites with an N-glycosylation potential >0.5, as predicted according to the Server NetNGlyc 1.0 [64] in V1 or V2 are indicated in the red and blue regions, respectively. Panel D. Representation of the V1V2 region in the V1 or in the V2 chimeras according to the structure of McLellan et al. [31]. The representation is as in Figure 1A. The colored part of the drawing corresponds to the parts that were replaced.