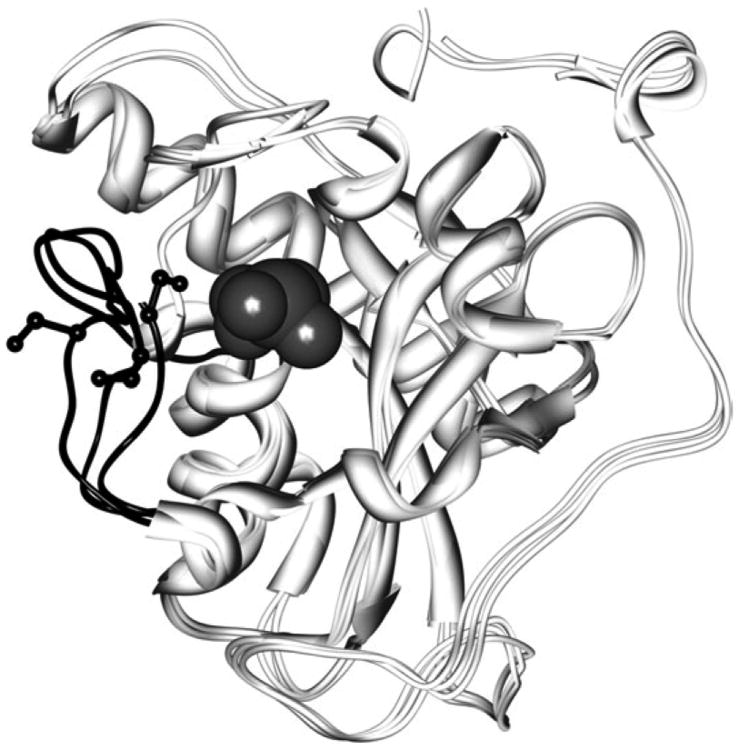
Figure 2.
Comparison of Msr conformations illustrates conformational variability. Three structures of MsrA are superimposed (1fva, 1fvg, and 1ff3), illustrating the conformational variability that exists in the C-terminal region forming an active site loop (shown in black ribbon). This conformational variability affects the pKa of the modifiable cysteines (gray van der Waals spheres). The modifiable cysteines are found in the same conformation in each structure (gray van der Waals spheres), but the nearby active site loop contains another cysteine, found in varying conformations (side chains in black ball and stick representation).