Abstract
Prebiotic fibres have been proposed to promote weight loss and lower serum cholesterol; however, the mechanisms are not fully understood. The aim of the present research was to identify possible mechanisms through which prebiotic fibres improve serum lipids. Lean and obese JCR:La-cp rats aged 8 weeks consumed one of three diets supplemented with 0, 10 or 20 % prebiotic fibre for 10 weeks. Rats were anaesthetised and a fasting blood sample was taken for lipid analysis. Real-time PCR was used to determine gene expression for cholesterol and fatty acid regulatory genes in liver tissue. Liver and caecal digesta cholesterol and TAG content were quantified. Both doses of prebiotic fibre lowered serum cholesterol levels by 24 % in the obese hyperlipidaemic rats (P<0·05). This change was associated with an increase in caecal digesta as well as an up-regulation of genes involved in cholesterol synthesis and bile production. Additionally, there was a 42 % reduction in TAG accumulation in the liver of the obese rats with 10 % prebiotic diet (P<0·05); however, no change in liver fatty acid synthase (FAS). Prebiotic fibres appear to lower cholesterol levels through increased cholesterol excretion in the form of bile and inhibit the accumulation of TAG in the liver through a mechanism unrelated to FAS. These effects appear to be limited to the obese model and particularly the 10 % dose. The present work is significant as it provides insight into the mechanisms of action for prebiotic fibres on lipid metabolism and furthers the development of dietary treatments for hypercholesterolaemia.
Keywords: Inulin and oligofructose, Lipid metabolism, Liver TAG, Cholesterol content, Gene expression
Individuals with obesity often display hyperlipidaemia, hypercholesterolaemia and insulin resistance(1). Therapies targeting both excessive body fat stores as well as lipid parameters may be beneficial, as they have the potential to simultaneously treat obesity and its comorbidities.
The effects of a variety of dietary fructans on lipid metabolism have been tested in rodent studies. Improvements in cholesterol, TAG, hepatic steatosis and plaque formation have been demonstrated when either regular chow or high-fat diets are supplemented with prebiotic fibre(2 – 7). Prebiotic supplementation has also been examined in pigs, where a decrease in plasma total cholesterol (TC), LDL cholesterol and TAG was reported(8). These studies generally support a conclusion that prebiotic fibres have beneficial effects on lipid metabolism in animal models(9). Studies in human subjects, however, tend to be conflicting and it has been suggested that the effectiveness of prebiotics is dependent on lipid status, with positive outcomes more commonly reported in individuals with hyperlipidaemia or hypercholesterolaemia(9).
Prebiotic fibres are considered to be both soluble as well as fermentable; however, they are unique with respect to their solubility as they do not gel in the gut nor modify viscosity(4). These characteristics are notable in that the traditionally proposed mechanism by which soluble fibre acts, namely complexing with dietary fat to form a stable gel-like emulsion that prevents pancreatic lipase from hydrolysing fat(10), does not apply. Consequently, it has been proposed that effects on lipid metabolism are due to inhibition of de novo fatty acid synthesis(11,12). One theory is that prebiotic fibres are able to downregulate hepatic lipogenic enzymes, specifically fatty acid synthase (FAS), through increased production of the SCFA propionate(9).
Prebiotic fibres likely have unique modes of action with respect to cholesterol metabolism specifically related to their fermentability and modulation of the microflora. Previously, SCFA have been implicated in cholesterol metabolism. In rodents, inulin supplementation results in an increase in SCFA in the caecum, as compared to a control diet(2). Concentrations measured in the portal vein suggest that the liver is exposed to high concentrations of propionic acid. The authors conclude that the availability of propionic acid to the liver depresses cholesterolaemic responses(2). In vitro, in rat hepatocytes, propionate competitively inhibits acetate thereby attenuating its effects on the cholesterogenic pathway; however, the effects were much less pronounced in human hepatocytes, suggesting the mechanism may be species specific(9,13). Alterations in gut microflora may also influence cholesterol absorption. Addition of oligofructose and inulin to the diet is associated with increased bifidobacteria and lactobacilli(14,15). In vitro, Lactobacillus acidophilus and Bifidobacterium bifidum strains demonstrate enhanced bile acid deconjugation, thereby enhancing bile acid and cholesterol precipitation. The physiological effectiveness of this process is contentious however, as a low pH has been proposed as a requirement for the co-precipitation of cholesterol(16). A similar mechanism has also been proposed for the hypocholesterolaemic effect of L. acidophilus on serum cholesterol in pigs(17). Generally, acidification of the caecal contents renders bile acids and cholesterol insoluble(2,18), and, in vitro, oligofructose supplementation has been shown to bring the pH of faecal cultures down to 3·7(15). Theoretically, if these effects translated in vivo, less bile would be reabsorbed in the gut, serum concentrations would decrease and less bile acid would reach the liver through enterohepatic circulation. The liver would compensate by increasing bile production from cholesterol further lowering serum cholesterol levels(17,19). Recent microarray profiling of hepatic genes in rodents fed psyllium fibre supports this theory as the authors report increased expression of several genes involved in cholesterol synthesis(20).
Given the unique characteristics of prebiotic fibres, a detailed examination of genes regulating hepatic cholesterol and fatty acid metabolism, cholesterol excretion and liver lipid content is warranted to explain their lipid-modulating effects. Furthermore, a dose response to the prebiotic fibres, oligofructose and inulin, has not previously been performed and is examined here.
Experimental methods
Animal model
Male lean (+/?) and obese (cp/cp) JCR:La-cp rats 8 weeks of age were obtained from the colony of Dr Spencer Proctor (University of Alberta, Edmonton, AB, Canada). The obese cp/cp rat lacks a functional leptin receptor and exhibits hepatic steatosis and elevated serum TAG and cholesterol levels(21). Animals were housed in a temperature and humidity controlled room with a 12 h light–dark cycle. All tissues and blood were collected in the fasted state. The protocol was approved by the University of Calgary Animal Care Committee and conformed to procedures set forth for the Care and Use of Laboratory Animals.
Dietary intervention
At 8 weeks of age, the animals were randomly allocated to a control (0 % prebiotic fibre), fibre (10 % prebiotic fibre) or high-fibre (20 % prebiotic fibre) w/w diet for 10 weeks. Groups included: lean control; lean fibre; lean high fibre; OC, obese control; OF, obese fibre; OHF, obese high fibre (n 8 rats). Experimental diets were formulated based on the AIN-93M diet (Dyets, Inc., Bethlehem, PA, USA) with the addition of a 1:1 mix of inulin and oligofructose (the mixture resembles the commercially available Orafti Synergy 1™ and was prepared by mixing equal amounts of Raftiline HP and Raftilose P95 supplied by Quadra Chemicals Ltd, Burlington, ON, Canada) to create diets that were 10 or 20 % prebiotic fibre by weight (Table 1). The standard AIN-93M diet was used as the control. The diets were formulated to match protein, fat and micronutrient content as closely as possible. The prebiotic fibre diets have lower energy contents due to the energy-diluting effect of the prebiotic fibre; however, a change in simple sugars was avoided by adjusting maize starch rather than sucrose to make up the difference. Food intake was recorded daily.
Table 1.
Composition of the experimental diets
| Control* (g/kg) | 10 % prebiotic fibre (g/kg) | 20 % prebiotic high fibre (g/kg) | |
|---|---|---|---|
| Maize starch/glucose | 46·69 | 39·45 | 33·51 |
| Casein | 14·04 | 13·11 | 12·03 |
| Dextrinised maize starch | 15·54 | 14·51 | 13·32 |
| Sucrose | 10·03 | 9·36 | 8·59 |
| Soyabean oil | 4·01 | 3·75 | 3·44 |
| AIN-93M-MX | 3·51 | 3·48 | 3·39 |
| AIN-93-VX | 1·00 | 0·99 | 0·96 |
| Choline bitartrate | 0·18 | 0·18 | 0·17 |
| L-Cystine | 0·25 | 0·25 | 0·24 |
| Alphacel | 5·01 | 4·97 | 4·83 |
| Fibre source† | 0·00 | 9·94 | 19·53 |
| Digestible energy (kJ/g) | 15·90 | 14·81 | 13·81 |
Based on AIN-93 (Dyets, Inc.) diet for the maintenance of adult rats.
1:1 Blend of inulin (supplied as Orafi Raftiline HP by Quadra Chemicals Ltd) and oligofructose (supplied as Orafti Raftilose by Quadra Chemicals Ltd).
Body composition
Body weight was recorded weekly using an electronic scale. Body composition was measured 1 d before sacrifice by a dual energy X-ray absorptiometry scan in conjunction with the use of Hologic QDR software for small animals (QDR 4500; Hologic, Inc., Bedford, MA, USA).
Liver cholesterol and TAG
An electronic scale accurate to three decimal places was used to measure mass. For cholesterol quantification, approximately 10 mg of wet liver tissue was used for the extraction of liver cholesterol and was sonicated in 200 μl of choloroform–Triton X-100 solution. The extract was spun at top speed in a microcentrifuge for 10 min. The organic phase was collected and dried at 50°C followed by a second 30 min vacuum dry. The dried lipids were dissolved in Triton X-100 and 200 μl of cholesterol reaction buffer. For determination of cholesterol, a colorimetric assay (BioVision Research Products, Mountain View, CA, USA) was used. Briefly, the cholesterol standard was diluted to create a standard curve. Reaction mix was added to the standard and test samples. The reaction was incubated for 1 h at 37°C. Optical densities of the samples were measured at 570 nm. For calculations, the background was first subtracted and then cholesterol concentration was generated based on the standard curve.
For TAG, approximately 25 mg of wet liver tissue were weighed and the TAG extracted with a KOH–EtOH solution. Samples were placed at 70°C for 1 h and then allowed to rest overnight. The volume was brought up to 500 μl with 2M-Tris–HCl. Samples were diluted 1:5 with the Tris–HCl. Quantification of the TAG was done colorimetrically with a TAG (glycerol-3-phosphate oxidase) liquid reagent set (Point Scientific, Inc., Lincoln Park, MI, USA). One millilitre of glycerol-3-phosphate oxidase was added to a tube for each standard or sample and heated to 37°C for 5 min. Standard or sample was added to the glycerol-3-phosphate oxidase and heated for another 5 min; 200 μl of each were added to a plate and read at 500 nm. TAG content in mmol/l was determined based on the linear curve (Protocol provided by DH Wasserman’s Lab, Vanderbuilt University School of Medicine, Nashville, TN, USA)(22).
Caecal TAG and cholesterol
Caecal digesta samples were dried overnight in a freeze drier. Extraction of cholesterol and TAG from the caecal samples and calculations were performed as described earlier.
Serum lipid analysis
To determine serum lipid profiles, 0·6 ml of blood was collected from each rat into a serum tube. The blood was centrifuged at 1600 g for 15 min at 4°C and the serum was collected for analysis. Samples were sent to Calgary Laboratory Services (Calgary, AB, Canada) for quantification of TC, HDL cholesterol and TAG. LDL cholesterol levels were calculated by subtracting HDL cholesterol and (TAG/5) from TC.
RNA isolation and real-time PCR
RNA isolation and real-time PCR procedures were completed as previously described(23). Briefly, total RNA was extracted with TRIzol reagent and reverse transcription was performed to generate complementary DNA. The resultant complementary DNA was amplified using primers synthesised by University of Calgary Core DNA Services (Calgary, AB, Canada). Primer sequences are available upon request from the authors. A DNA iCycler was used for the PCR, and the resulting melt curve showed the melting point of the PCR product of interest. Actin primers were included as an internal control. In both the lean and obese groups, the control diet was used as the control condition. For comparisons between the lean and obese groups, the lean group was used as the control. Fold change from control was calculated using the comparative threshold cycle time method(24).
Statistical analysis
All data are presented as mean (SE). Changes in physiology, serum lipids and caecal digesta were determined using two-factor ANOVA (with diet and genetic group (lean v. obese) as variables) with a Holm–Sidak test for multiple comparisons. Changes in gene expression between the diets within the lean and obese groups were analysed using one-way ANOVA with a Bonferroni adjustment for multiple comparisons. Data were analysed using SigmaStat 3.5 (Systat Software, Inc., San Jose, CA, USA) software and STATA 8 (STATA Corporation, College Station, TX, USA). A result was considered significant when P≤0·05.
Results
Liver characteristics
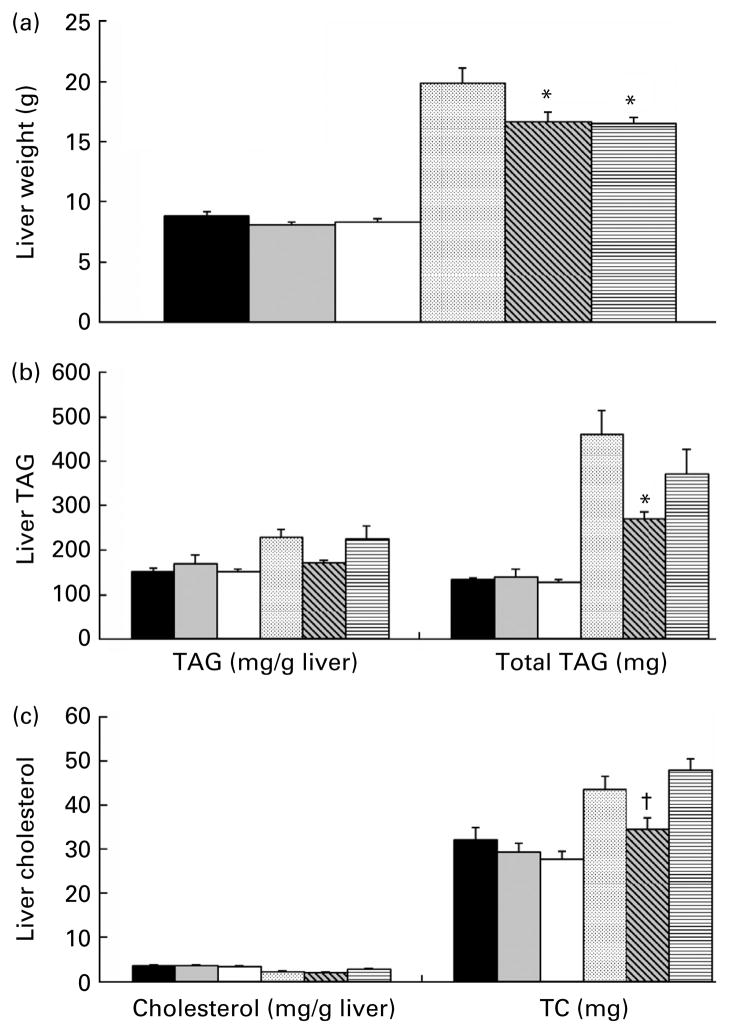
Energy intake was reduced (P<0·05) with prebiotic supplementation compared to control across all groups except lean fibre group (Table 2). The lower body weight and fat mass observed with increasing doses of fibre, however, were not significantly different (P>0·05). Caecal weight was dose dependently increased with increasing doses of fibre (P<0·05). Total liver weight decreased in the OF (P<0·01) and OHF (P<0·01) groups compared to OC group (Fig. 1(a)). Liver TAG expressed per gram of tissue was unchanged with prebiotic supplementation. Total TAG, however, which takes into account liver weight and TAG content, showed a significant group by diet interaction effect (P<0·05; Fig. 1(b)). Separate analysis of the lean and obese groups indicated that the combination of decreased liver mass and decreased TAG/g tissue resulted in a 42 % decrease in total liver TAG in the OF group (P=0·03) and a 20 % decrease in the OHF group (P=0·57) compared to OC group (Fig. 1(b)). Liver cholesterol expressed per gram of tissue did not differ across groups (Fig. 1(c)). There was, however, a significant group by diet interaction detected for total liver cholesterol (P<0·05). Given the reduced liver size, TC was lower in the OF group compared to both OC (P=0·06) and OHF (P=0·01) groups. There was no difference between OHF v. OC groups.
Table 2.
Physical characteristics of lean and obese JCR:LA-cp rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets (Mean values with their standard errors for n 8 except obese high fibre (OHF) group n 7)
| LC
|
LF
|
LHF
|
OC
|
OF
|
OHF
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Energy intake (MJ) | 16·39 | 0·2 | 15·4 | 0·2 | 14·7* | 0·3 | 27·0 | 0·6 | 25·4* | 0·5 | 24·9* | 0·6 |
| Body wt (g) | 345·6 | 5·0 | 340·6 | 8·4 | 334·4 | 3·7 | 567·2 | 9·5 | 563·2 | 8·5 | 561·5 | 10·9 |
| Fat mass (%) | 7·6 | 0·2 | 5·9 | 1·0 | 5·6 | 0·6 | 55·1 | 1·1 | 54·3 | 1·5 | 52·6 | 1·2 |
| Caecal wt† (g) | 2·7 | 0·4 | 6·9* | 0·5 | 9·3*‡ | 0·5 | 2·1 | 0·2 | 5·0* | 0·4 | 8·4*‡ | 0·9 |
| Caecal TAG (mg/g) | 27·5 | 2·6 | 24·4 | 2·6 | 23·7 | 2·6 | 33·9 | 2·6 | 36·4 | 2·7 | 20·8*‡ | 2·7 |
| Caecal cholesterol (mg/g) | 13·0 | 2·7 | 15·1 | 2·7 | 14·7 | 2·7 | 19·0 | 2·7 | 26·7 | 2·7 | 14·9‡ | 2·9 |
LC, lean control; LF, lean fibre; LHF, lean high fibre; OC, obese control; OF, obese fibre; OHF, obese high fibre.
Mean values were significantly different from control diet within lean and obese groups.
Caecal weight refers to the caecal tissue and the wet contents. Caecal TAG and cholesterol are expressed as mg/g total caecal weight (tissue + contents).
Mean values were significantly different between the 10 and 20 % fibre diets within lean and obese groups as determined by a two-way ANOVA with a Holm–Sidak adjustment for multiple comparisons (P≤0·05).
Fig. 1.
Liver characteristics for the lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Panel (a) depicts total liver weight. Panel (b) depicts liver TAG content. The series on the left shows TAG content per g of wet liver tissue and the series on the right shows total TAG content of the liver. Total TAG content was calculated by multiplying TAG/g of tissue by total liver weight. For ease of presentation, total liver TAG content was reduced by a factor of 10. Actual values from left to right are 1343·0, 1393·7, 1276·7, 4626·5, 2701·2 and 3719·2 mg. Panel (c) depicts liver cholesterol content. The series on the left shows cholesterol content per gram of wet liver tissue and the series on the right shows total cholesterol (TC) content of the liver. TC content was calculated by multiplying cholesterol per gram of tissue by total liver weight. Data are mean (SE); n 8 except obese high fibre (OHF) group n 7. * Mean values were significantly different from control diet within lean or obese groups. † Mean values were signifi-cantly different between the 10 % and 20 % fibre diets within lean or obese groups as determined by ANOVA with Holm–Sidak adjustment for multiple comparisons or a one-way ANOVA with Bonferroni adjustment where there was a significant diet and group interaction (P≤0·05). LC (■), lean control; LF (
 ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC (
 ), obese control; OF (
), obese control; OF (
 ); obese fibre; OHF (▤), obese high fibre.
); obese fibre; OHF (▤), obese high fibre.
Serum lipids
There was a significant group by diet interaction for serum TC (Fig. 2). When the groups were analysed separately, both doses of prebiotic fibre resulted in a 24 % decrease in TC (P=0·05 and 0·08 for OF and OHF groups, respectively). LDL cholesterol was significantly lower in OHF group compared to OC group (P<0·05). There was no effect on TAG in lean or obese animals, and HDL cholesterol was reduced in both the OF (P<0·01) and OHF (P=0·02) groups compared to OC group. There was a significant group by diet interaction for the ratio of total:HDL cholesterol; however, analysis in the individual groups did not indicate a diet effect.
Fig. 2.
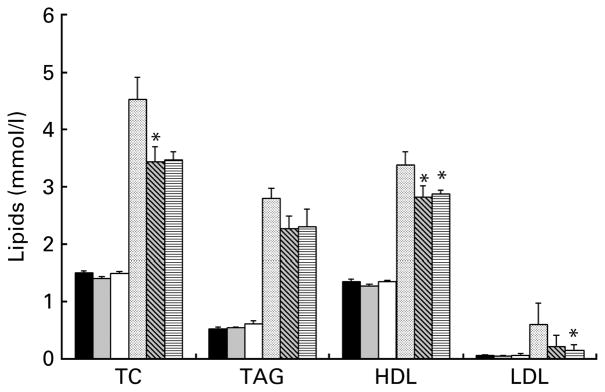
Serum lipid profiles for lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Results are mean (SE), n 8 except for LDL where some samples were below detection (n 4–6). * Mean values were significantly different from the control diet within the respective lean and obese groups as determined by ANOVA with Holm–Sidak adjustment for multiple comparisons or a one-way ANOVA with Bonferroni adjustment where there was a significant diet by group interaction (P≤0·05). LC (■), lean control; LF (
 ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC (
 ), obese control; OF (
), obese control; OF (
 ); obese fibre; OHF (▤), obese high fibre; TC, total cholesterol.
); obese fibre; OHF (▤), obese high fibre; TC, total cholesterol.
Gene expression
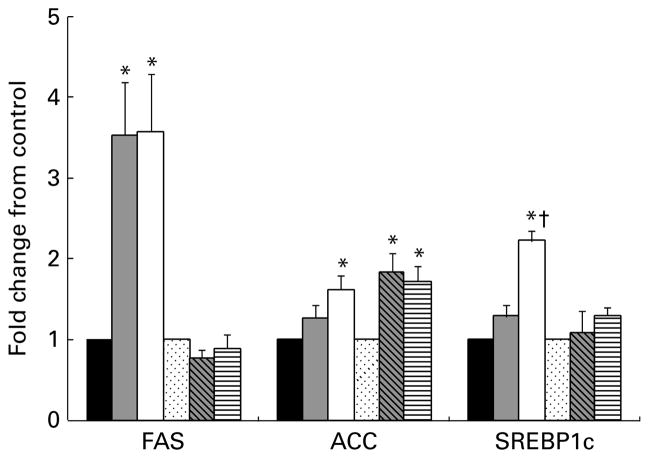
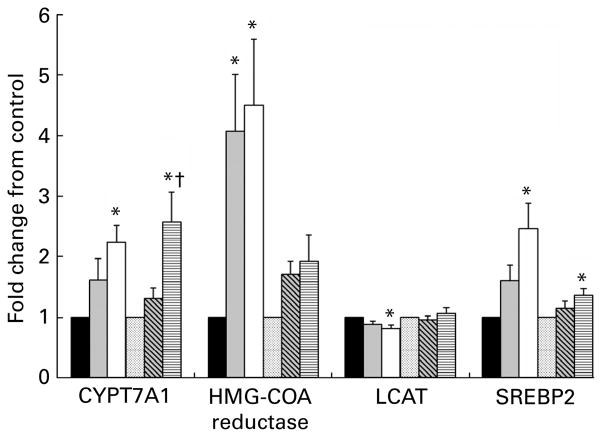
With respect to fatty acid metabolism, there was a 3·5-fold up-regulation of FAS in the lean fibre and lean high fibre groups compared to lean control group (Fig. 3). This coincided with an up-regulation of sterol regulatory element-binding protein 1c, a known transcriptional regulator of FAS. Furthermore, acetyl-CoA carboxylase-α mRNA was increased approximately twofold in both the lean and obese groups on the 20 % fibre diet and in the 10 % obese group (Fig. 3). With respect to cholesterol metabolism, a fourfold increase in 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase was noted in the lean group with the 10 % and 20 % fibre doses compared to control (Fig. 4). The regulatory enzyme sterol regulatory element-binding proteins 2 also matched this up-regulation (P<0·05). A similar but non-significant pattern for HMG-CoA was seen in the OHF group. Conversely, lecithin:cholesterol acyltransferase was down regulated 19 % in the lean high fibre group compared to lean control group, while cholesterol 7-α-hydroxylase (CYPT7A1), an enzyme involved in bile synthesis, was increased twofold with fibre supplementation in both the lean and obese 20 % fibre groups (Fig. 4). There was also a sevenfold increase in FAS mRNA expression in the obese group compared to the lean group (P<0·001). When stratified by diet, FAS mRNA expression increased in the obese groups compared to the lean groups on the control (P<0·001) and fibre (P<0·01) diets.
Fig. 3.
Liver mRNA levels of genes primarily associated with fatty acid metabolism for lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Results represent the mean fold change (SE) compared to control, n 8 except obese high fibre (OHF) group n 7. * Mean values were significantly different from the control diet within lean and obese groups † Mean values were significantly different between the 10 % and 20 % fibre diets within lean and obese groups as determined by one-way ANOVA with Bonferroni adjustment (P≤0·05). LC (■), lean control; LF (
 ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC (
 ), obese control; OF (
), obese control; OF (
 ), obese fibre; OHF (▤), obese high fibre; FAS, fatty acid synthase; ACC, acetyl-CoA carboxy-lase-α; SREBP1c, sterol regulatory element-binding protein 1c.
), obese fibre; OHF (▤), obese high fibre; FAS, fatty acid synthase; ACC, acetyl-CoA carboxy-lase-α; SREBP1c, sterol regulatory element-binding protein 1c.
Fig. 4.
Liver mRNA levels of genes primarily associated with cholesterol metabolism for lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Results represent the mean fold change (SE) compared to control, n 8 except obese high fibre (OHF) group n 7. * Mean values were significantly different from the control diet within lean and obese groups. † Mean values were significantly different between the 10 and 20 % fibre diets within lean and obese groups as determined by one-way ANOVA with Bonferroni adjustment (P≤0·05). LC (■), lean control; LF (
 ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC (
 ), obese control; OF (
), obese control; OF (
 ), obese fibre; OHF (▤), obese high fibre; CYPT7A1, cholesterol 7-α-hydroxylase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LCAT, lecithin:cholesterol acyltransferase; SREBP2, sterol regulatory element-binding protein 2.
), obese fibre; OHF (▤), obese high fibre; CYPT7A1, cholesterol 7-α-hydroxylase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LCAT, lecithin:cholesterol acyltransferase; SREBP2, sterol regulatory element-binding protein 2.
Caecal TAG and cholesterol
Analysis of dried caecal contents revealed a significant group by diet interaction (P<0·05). Caecal TAG was significantly lower in OHF group compared to OC and OF groups (P<0·001). Taken together with the increased caecal weight, comprised of the mass of the caecal tissue and its wet contents (Table 2), greater TAG excretion would be likely due to the greater volume of caecal digestion noted with fibre supplementation. In lean animals, decreases in dry caecal TAG/g with increasing fibre were not significantly different. Caecal cholesterol levels were significantly lower in the OHF group compared to the OF group (P<0·01).
Discussion
The goal of the present study was to determine the dose–response effect of prebiotic fibre supplementation on lipid metabolism. The major finding is that prebiotic fibres reduce serum cholesterol levels through lower cholesterol absorption and modulation of hepatic gene expression. Secondary findings suggest that prebiotic fibres are able to reduce liver TAG accumulation.
The present study provides evidence that prebiotic fibre supplementation lowers total serum cholesterol in a hyper-cholesterolaemic rat model. These results are in support of several other reports of lower cholesterol levels with prebiotic fibre intake(4,5,7). The present results suggest that the reduction in serum cholesterol is primarily due to a sequence of events consisting of (1) decreased cholesterol absorption from the gut; (2) reduced hepatic exposure to bile salts; (3) enhanced cholesterol production by the liver and/or cholesterol uptake from the plasma; (4) increased bile production; (5) greater cholesterol excretion from the body in the form of bile. Direct measurement of total dried caecal contents was not possible; however, we do report a significant increase in caecal size, including tissue and wet contents, and others report an increase of 330 % in caecal digesta with prebiotic fibre(25), which would ultimately increase the amount of cholesterol excreted from the body.
This theory is further strengthened by our gene expression data. Firstly, an increase in the expression of HMG-CoA reductase, an enzyme that regulates the conversion of HMG-CoA to mevalonate, the rate-limiting step in cholesterol synthesis(26), was increased with prebiotic fibre. Furthermore, the sterol regulatory element-binding proteins, which are considered to be regulatory factors for HMG-CoA reductase(27), were upregulated. A possible explanation for this outcome is that genes involved in cholesterol synthesis are upregulated by reductions in bile absorption due to the prebiotic fibre. Support for bile salts as a regulatory product for HMG-CoA reductase has previously been demonstrated. Here, chronic biliary diversion stimulated HMG-CoA reductase, whereas taurocholate, a hydrophobic bile salt, inhibited HMG-CoA reductase suggesting bile salts can regulate HMG-CoA reductase through negative feedback(28). If these observations are extrapolated to this model, reduced uptake of bile from the gut would reduce hepatic exposure to bile salts thereby stimulating gene expression and consequently cholesterol production. Although increased cholesterol production would seem to contradict lower serum levels, it appears to coincide with increased cholesterol excretion in the form of bile.
Cholesterol can be incorporated into bile salts by CYPT7A1, the initial and rate-determining enzyme for bile synthesis(29). Newly synthesised cholesterol has been shown to be the preferred substrate for CYPT7A1 in rats(30). Conversely, a study in hamsters reported increased incorporation of preformed cholesterol into bile acids under physiological conditions(31). It is possible that the conflicting results are due to differences in lipid metabolism between the two species(32). We did not test for increases in hepatic LDL receptor mRNA; however, increases we observed in sterol regulatory element-binding protein would likely predict increased cholesterol uptake from the plasma given their involvement in the activation of the LDL receptor gene(27,33). Although the proportion of newly synthesised v. preformed cholesterol in bile salts remains unclear, both are incorporated in some manner(30,31). As increased CYPT7A1 mRNA was noted in the present study, it is reasonable to assume that increased amounts of de novo and preformed cholesterol are being incorporated into bile and subsequently excreted by the prebiotic fibre groups.
Further support for this model comes from the regulation of CYPT7A1. If the assumption of decreased bile absorption from the gut with prebiotic fibre intake is correct, reduced liver exposure to bile salts would occur. This in turn would affect bile production, as bile salts are reported to regulate CYPT7A1 through a negative feedback mechanism. Indeed, CYPT7A1 activity, mRNA and transcription rates have been reported to decrease with exposure to bile acids(34). Specifically, high levels of bile salts reaching the liver through the portal vein suppress CYPT7A1 and consequently bile production, whereas biliary diversion and binding resins stimulate CYPT7A1(28,32). Here, increased CYPT7A1 is reported suggesting increased bile production, an effect likely stimulated by reduced bile absorption initially. In summary, liver and caecal analyses support a chain of events whereby prebiotic fibres inhibit bile absorption, thereby leading to alterations in liver metabolism, of which the end product is increased cholesterol secretion in the form of bile.
A variety of other fibres have been proposed to exert their hypocholesterolaemic effects through inhibition of bile absorption(32). Increased cholesterol metabolism and secretion in the form of bile acids have been noted with pectin and psyllium fibres. Matheson et al. (35) report increased CYPT7A1 mRNA with both psyllium and pectin feeding and note that the magnitude correlates with the increased bile acid pool sizes reported in their previous experiments(36). Psyllium has also been shown to increase CYPT7A1 mRNA and faecal bile acids in rats(37). Finally, analysis of cholesterol metabolism in psyllium fibre-fed mice showed an up-regulation of HMG-CoA reductase and CYPT7A1(20).
Prebiotic fibres likely inhibit bile salt absorption differently than other soluble type fibres due to their lack of viscosity. Possible mechanisms include enhanced bile acid deconjugation(16), cholesterol assimilation into the bacterial cells(38 – 40) and acidification of the caecal contents(2,18).
Lecithin:cholesterol acyltransferase has a role in the esterification of free cholesterol, thus generating a cholesteryl ester, which can be transferred to the HDL particle resulting in an increase in HDL particle size(41,42). A decrease in hepatic lecithin:cholesterol acyltransferase gene expression could be explained by the overall reduction in cholesterol and the slightly lower serum HDL cholesterol levels. Differences between the lean and obese rats likely reflect differences in serum lipids with a larger increase in HMG-CoA reductase in the lean rats in an effort to maintain minimal serum cholesterol levels. Dietary differences, specifically the increase in cholesterol accumulation in the liver in the OHF groups as compared to the OF group, remain unexplained; however, may be due to the increase in SCFA or alterations in the balance of propionate to acetate. The SCFA acetate has been reported to stimulate cholesterol production(9), and oligofructose has been reported to increase acetic acid to a greater extent than propionic acid in vitro (15). Therefore, increased cholesterol production could reflect greater bile acid excretion in combination with increased acetate production.
Prebiotic fibre supplementation has also been reported to affect TAG metabolism. In rats, oligofructose supplementation decreased serum and hepatic TAG. These effects were attributed to reduced de novo fatty acids synthesis as FAS levels were attenuated(12). We report a trend towards decreased liver weight and TAG content leading to overall lower liver TAG in our obese model. Conversely, there was no change in FAS mRNA levels in the obese animals. Similar null effects in genetically obese Zucker rats have been reported(43). Acetyl-CoA carboxylase-α gene expression in the liver was increased; however, the relevance of this in the absence of increased FAS and TAG accumulation is debatable, as FAS is considered to be an essential enzyme for fatty acid synthesis(44). Further research is needed to identify the mechanisms for decreased TAG in the absence of alterations in liver fatty acid gene expression in this obesity model. Conversely, in the lean model, FAS as well as one of its regulatory factors, sterol regulatory element-binding protein 1c(44), was increased. This increase in FAS is contrary to several previous reports of reduced FAS with prebiotic supplementation(12,45); however, similar results showing increased FAS mRNA have also been reported with long-term (10 week) supplementation with psyllium fibre(20). Importantly, no increase in liver size or TAG accumulation was reported in the present study. Additionally, increased TAG storage in adipose is unlikely as, if anything, there was reduced fat mass noted with supplementation (Table 2). These results indicate that increased FAS is not directly linked to TAG accumulation in the present study. Further research is required to explain the up-regulation of liver FAS in this lean model. A possible explanation would be that the prebiotic fibre increases TAG excretion, and the increase in FAS reflects an increase in fatty acid production to maintain serum and liver TAG levels. However, FAS gene expression increased in the obese groups compared to the lean group suggesting obesity is associated with increased de novo TAG production. This is an important observation as it highlights the importance of addressing differences between the lean and obese states and potential varied responses to the same dietary intervention.
In conclusion, prebiotic fibre supplementation appears to be beneficial in the hyperlipidaemic and hypercholesterolaemic states. Serum cholesterol levels are reduced likely due to increased cholesterol excretion. TAG accumulation, particularly in the liver, may also be improved; however, the mechanism(s), specifically the importance of FAS, remains elusive. The present research is significant as it helps to unravel the mechanism of action for prebiotic fibres. Several studies indicate that prebiotic supplementation may be beneficial for human subjects with dyslipidaemia; however, the mechanisms remain unclear and the results are inconsistent(9). A further understanding of the mechanisms of action and dose-dependent responses is crucial to optimise the benefits of prebiotic fibres. The present animal study provides insight into possible mechanisms of action in human subjects and suggests that different actions and outcomes may result depending on body weight status and underlying disease state. Additionally, it is the first experiment to assess the effects of increasing amounts of these fibres to determine optimal dosages. Future research should focus on the unique mechanisms of prebiotic fibre including microbial modifications. If it can be identified that prebiotic fibres function differently than other types of soluble fibres, the effects of fibre combinations should be explored.
Acknowledgments
The present work was supported by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. The authors declare no conflict of interest. The authors would also like to thank Kristine Lee for her assistance with the laboratory work. J. A. P. coordinated and carried out the animal procedures, analysed the data and wrote the initial draft. R. A. R. designed and obtained funding for the study and contributed to the final version of the manuscript.
Abbreviations
- CYPT7A1
cholesterol 7-α-hydroxylase
- FAS
fatty acid synthase
- HMG-CoA
3-hydroxy-3-methylglutaryl-CoA
- OC
obese control
- OF
obese fibre
- OHF
obese high fibre
- TC
total cholesterol
References
- 1.Kissebah AH, Freedman DS, Peiris AN. Health risks of obesity. Med Clin North Am. 1989;73:111–138. doi: 10.1016/s0025-7125(16)30695-2. [DOI] [PubMed] [Google Scholar]
- 2.Levrat M-A, Favier M-L, Moundras C, et al. Role of dietary propionic acid and bile acid excretion in the hypo-cholesterolemic effects of oligosaccharides in rats. J Nutr. 1994;124:531–538. doi: 10.1093/jn/124.4.531. [DOI] [PubMed] [Google Scholar]
- 3.Cani PD, Neyrinck AM, Maton N, et al. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res. 2005;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 4.Urias-Silvas JE, Cani PD, Delmee E, et al. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br J Nutr. 2008;99:254–261. doi: 10.1017/S0007114507795338. [DOI] [PubMed] [Google Scholar]
- 5.Rault-Nania MH, Gueux E, Demougeot C, et al. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006;96:840–844. doi: 10.1017/bjn20061913. [DOI] [PubMed] [Google Scholar]
- 6.Daubioul C, Rousseau N, Demeure R, et al. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J Nutr. 2002;132:967–973. doi: 10.1093/jn/132.5.967. [DOI] [PubMed] [Google Scholar]
- 7.Rozan P, Nejdi A, Hidalgo S, et al. Effects of lifelong intervention with an oligofructose-enriched inulin in rats on general health and lifespan. Br J Nutr. 2008;100:1192–1199. doi: 10.1017/S0007114508975607. [DOI] [PubMed] [Google Scholar]
- 8.Liong MT, Dunshea FR, Shah NP. Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. Br J Nutr. 2007;98:736–744. doi: 10.1017/S0007114507747803. [DOI] [PubMed] [Google Scholar]
- 9.Delzenne NM, Williams CM. Prebiotics and lipid metabolism. Curr Opin Lipidol. 2002;13:61–67. doi: 10.1097/00041433-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Artiss JD, Brogan K, Brucal M, et al. The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism. 2006;55:195–202. doi: 10.1016/j.metabol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Williams CM. Effects of inulin on lipid parameters in humans. J Nutr. 1999;129:1471S–1473S. doi: 10.1093/jn/129.7.1471S. [DOI] [PubMed] [Google Scholar]
- 12.Kok N, Roberfroid M, Delzenne N. Dietary oligofructose modifies the impact of fructose on hepatic tri-acylglycerol metabolism. Metabolism. 1996;45:1547–1550. doi: 10.1016/s0026-0495(96)90186-9. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Vonk RJ, Slooff MJH, et al. Differences in propionate-induced inhibition of cholesterol and triacylglycerol synthesis between human and rat hepatocytes in primary culture. Br J Nutr. 1995;74:197–207. doi: 10.1079/bjn19950123. [DOI] [PubMed] [Google Scholar]
- 14.Gibson GR, Beatty ER, Wang X, et al. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 15.Pompei A, Cordisco L, Raimondi S, et al. In vitro comparison of the prebiotic effects of two inulin-type fructans. Anaerobe. 2008;14:280–286. doi: 10.1016/j.anaerobe.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Klaver FA, van der Meer R. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl Environ Microbiol. 1993;59:1120–1124. doi: 10.1128/aem.59.4.1120-1124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Rodas BZ, Gilliland SE, Maxwell CV. Hypocholes-terolemic action of Lactobacillus acidophilus ATCC 43121 and calcium in swine with hypercholesterolemia induced by diet. J Dairy Sci. 1996;79:2121–2128. doi: 10.3168/jds.S0022-0302(96)76586-4. [DOI] [PubMed] [Google Scholar]
- 18.Remesy C, Levrat MA, Gamet L, et al. Cecal fermentations in rats fed oligosaccharides (inulin) are modulated by dietary calcium level. Am J Physiol. 1993;264:G855–G862. doi: 10.1152/ajpgi.1993.264.5.G855. [DOI] [PubMed] [Google Scholar]
- 19.Pereira DI, Gibson GR. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit Rev Biochem Mol Biol. 2002;37:259–281. doi: 10.1080/10409230290771519. [DOI] [PubMed] [Google Scholar]
- 20.Chan MY, Heng CK. Sequential effects of a high-fiber diet with psyllium husks on the expression levels of hepatic genes and plasma lipids. Nutrition. 2008;24:57–66. doi: 10.1016/j.nut.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Russell JC, Proctor SD, Kelly SE, et al. Pair feeding-mediated changes in metabolism: stress response and pathophysiology in insulin-resistant, atherosclerosis-prone JCR:LA-cp rats. Am J Physiol Endocrinol Metab. 2008;294:E1078–E1087. doi: 10.1152/ajpendo.90257.2008. [DOI] [PubMed] [Google Scholar]
- 22.Struys EA, Verhoeven NM, Jansen EE, et al. Metabolism of gamma-hydroxybutyrate to D-2-hydroxyglutarate in mammals: further evidence for D-2-hydroxyglutarate trans-hydrogenase. Metabolism. 2006;55:353–358. doi: 10.1016/j.metabol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Parnell JA, Reimer RA. Differential secretion of satiety hormones with progression of obesity in JCR:LA-corpulent rats. Obesity (Silver Spring) 2008;16:736–742. doi: 10.1038/oby.2007.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Busserolles J, Gueux E, Rock E, et al. Oligofructose protects against the hypertriglyceridemic and pro-oxidative effects of a high fructose diet in rats. J Nutr. 2003;133:1903–1908. doi: 10.1093/jn/133.6.1903. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 27.Vallett SM, Sanchez HB, Rosenfeld JM, et al. A direct role for sterol regulatory element binding protein in activation of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. J Biol Chem. 1996;271:12247–12253. doi: 10.1074/jbc.271.21.12247. [DOI] [PubMed] [Google Scholar]
- 28.Pandak WM, Vlahcevic ZR, Chiang JY, et al. Bile acid synthesis. VI. Regulation of cholesterol 7 alpha-hydroxylase by taurocholate and mevalonate. J Lipid Res. 1992;33:659–668. [PubMed] [Google Scholar]
- 29.Shefer S, Hauser S, Bekersky I, et al. Biochemical site of regulation of bile acid biosynthesis in the rat. J Lipid Res. 1970;11:404–411. [PubMed] [Google Scholar]
- 30.Bjorkhem I, Lewenhaupt A. Preferential utilization of newly synthesized cholesterol as substrate for bile acid biosynthesis. An in vivo study using 18O2-inhalation technique. J Biol Chem. 1979;254:5252–5256. [PubMed] [Google Scholar]
- 31.Scheibner J, Fuchs M, Hormann E, et al. Biliary cholesterol secretion and bile acid formation in the hamster: the role of newly synthesized cholesterol. J Lipid Res. 1994;35:690–697. [PubMed] [Google Scholar]
- 32.Gilardi F, Mitro N, Godio C, et al. The pharmacological exploitation of cholesterol 7[alpha]-hydroxylase, the key enzyme in bile acid synthesis: from binding resins to chromatin remodelling to reduce plasma cholesterol. Pharmacol Ther. 2007;116:449–472. doi: 10.1016/j.pharmthera.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Chen CW, Cheng HH. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J Nutr. 2006;136:1472–1476. doi: 10.1093/jn/136.6.1472. [DOI] [PubMed] [Google Scholar]
- 34.Pandak WM, Vlahcevic ZR, Heuman DM, et al. Effects of different bile salts on steady-state mRNA levels and transcriptional activity of cholesterol 7 alpha-hydroxylase. Hepatology. 1994;19:941–947. [PubMed] [Google Scholar]
- 35.Matheson HB, Colon IS, Story JA. Cholesterol 7 alpha-hydroxylase activity is increased by dietary modification with psyllium hydrocolloid, pectin, cholesterol and choles-tyramine in rats. J Nutr. 1995;125:454–458. doi: 10.1093/jn/125.3.454. [DOI] [PubMed] [Google Scholar]
- 36.Matheson HB, Story JA. Dietary psyllium hydrocolloid and pectin increase bile acid pool size and change bile acid composition in rats. J Nutr. 1994;124:1161–1165. doi: 10.1093/jn/124.8.1161. [DOI] [PubMed] [Google Scholar]
- 37.Buhman KK, Furumoto EJ, Donkin SS, et al. Dietary psyllium increases fecal bile acid excretion, total steroid excretion and bile acid biosynthesis in rats. J Nutr. 1998;128:1199–1203. doi: 10.1093/jn/128.7.1199. [DOI] [PubMed] [Google Scholar]
- 38.Noh DO, Kim SH, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci. 1997;80:3107–3113. doi: 10.3168/jds.S0022-0302(97)76281-7. [DOI] [PubMed] [Google Scholar]
- 39.Tahri K, Grill JP, Schneider F. Bifidobacteria strain behavior toward cholesterol: coprecipitation with bile salts and assimilation. Curr Microbiol. 1996;33:187–193. doi: 10.1007/s002849900098. [DOI] [PubMed] [Google Scholar]
- 40.Liong MT, Shah NP. Optimization of cholesterol removal by probiotics in the presence of prebiotics by using a response surface method. Appl Environ Microbiol. 2005;71:1745–1753. doi: 10.1128/AEM.71.4.1745-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vries R, Borggreve SE, Dullaart RP. Role of lipases, lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in abnormal high density lipoprotein metabolism in insulin resistance and type 2 diabetes mellitus. Clin Lab. 2003;49:601–613. [PubMed] [Google Scholar]
- 42.Borggreve SE, de Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33:1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 43.Daubioul CA, Taper HS, De Wispelaere LD, et al. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese Zucker rats. J Nutr. 2000;130:1314–1319. doi: 10.1093/jn/130.5.1314. [DOI] [PubMed] [Google Scholar]
- 44.Bennett MK, Lopez JM, Sanchez HB, et al. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J Biol Chem. 1995;270:25578–25583. doi: 10.1074/jbc.270.43.25578. [DOI] [PubMed] [Google Scholar]
- 45.Delzenne NM, Kok N. Effect of non-digestible fermentable carbohydrates on hepatic fatty acid metabolism. Biochem Soc Trans. 1998;26:228–230. doi: 10.1042/bst0260228. [DOI] [PubMed] [Google Scholar]