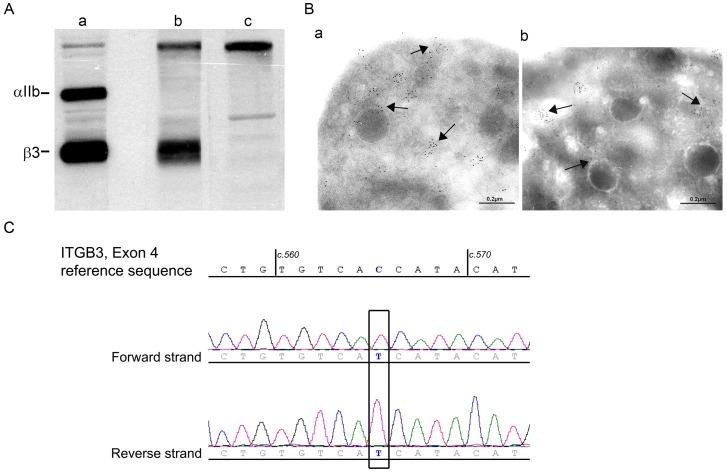
Figure 1. Initial studies characterizing the molecular defect of αIIbβ3 of the patient’s platelets.
A/Western blotting of αIIb and β3 in samples of SDS-soluble extracts of (a) control platelets (5μg protein), (b) the patient under study (60 μg) and (c) a second type I GT patient [21] with a large ITGB3 deletion preventing synthesis of β3 (60 μg). The integrin subunits were detected with a polyclonal antibody to αIIbβ3 with bound IgG located using 125I-labeled protein A. B/Immunogold labeling was performed on frozen-thin platelet sections using a pool of murine monoclonal antibodies specific for the αv subunit [8] and bound antibody located for platelets from (a) a control donor and (b) from the patient using a species-specific second antibody to mouse IgG adsorbed on 5 nm gold particles and electron microscopy. Arrows highlight the largely vesicular distribution of αvβ3. C/Direct sequencing of genomic DNA of the patient (forward and reverse strands) for exon 4 of ITGB3. The nucleotide concerned by the mutation is framed. The patient is homozygous for a c.565 C/T transition leading to a p.Pro189Ser substitution (P163S in the primary β3 structure nomenclature).