Abstract
Background:
Vitiligo is an acquired pigmentary disorder. In vivo reflectance confocal microscopy (RCM) reproducible imaging technique has already been reported to be useful in the diagnosis of other skin diseases.
Objective:
To define RCM features of vitiligo on different clinical stages.
Materials and Methods:
A total of 125 patients with a clinical diagnosis of vitiligo were included in this study. After informed consent, lesional skins of those vitiligo patients were characterized by using RCM. Five patients with inflammatory cell infiltration observed at the edge of skin lesions and another 5 patients without inflammatory cell infiltration were selected. Biopsies were performed at same sites of the RCM examination areas for histological and immune-histological analysis.
Results:
In the active stage of vitiligo, the RCM examination revealed that the bright dermal papillary rings presented at the dermoepidermal junction level in normal skin lost their integrity or totally disappeared, border between vitiligo lesion and normal skin became unclear, and highly refractile cells that referred to infiltrated inflammatory cells could be seen within the papillary dermis at the edge of the lesions. In the stable stage of vitiligo, the RCM showed a complete loss of melanin in lesional skin and a clear border between lesional and normal skin.
Conclusion:
A simple clinical examination with RCM may reliably and efficiently allow evaluation of the stability status of vitiligo lesions.
Keywords: Histopathology, immunohistological, reflectance confocal microscopy, vitiligo
Introduction
What was known?
RCM is a real-time, repetitive imaging tool that provides non-invasive images at a nearly histological resolution.
Vitiligo is the most common acquired hypopigmentary disorder, characterized by progressive loss of melanocytes. In vivo reflectance confocal microscopy (RCM) is a real-time, repetitive imaging tool that provides non-invasive images at a nearly histological resolution. The ability to evaluate a skin lesion microscopically in a non-invasive and real-time fashion has long been a goal for dermatologists and dermatopathologists. From August 2011 to September 2012, we used RCM to examine vitiligo patients to evaluate its role in clinical staging of vitiliginous lesions.
Materials and Methods
Subjects
This was a two-step study involving 125 patients with vilitigo. The patients were required not to receive treatment for vitiligo at least 2 months before the study. Their ages ranged from 1 to 75 years and the duration of disease was from 0.17 to 10 years. All patients were recruited prospectively from the Department of Dermatology over a period of 12 months. In the first stage of this study, the clinically significant vitiligo lesions from an even area were subjected to RCM examination. All patients provided informed consent for examination of their lesions by RCM.
In the second stage, the patients were divided into two groups on the basis of presence or absence of inflammatory cell infiltration at the edge of the lesional area. Five patients were then randomly selected from each group and biopsies were performed at same sites of the RCM examination areas for histological and immune-histological analysis.
In vivo reflectance confocal microscopy
A commercially available in vivo RCM device (Vivascope 1500; Lucid Inc, Rochester, NY, USA) was used for imaging. The instrument operated with a diode laser at an 830 nm wavelength and a laser power < 35 mW at tissue level. This system provided high-resolution images (approximately 1-2 μm in the lateral dimension and 4-5 μm in the axial ones) to a depth of 200 to 350 μm in vivo (from the epidermis down to the superficial reticular dermis). A 30 × water-immersion objective lens with a numerical aperture of 0.9 with water (refractive index, 1.33) as an immersion medium was used. To reduce motion artifacts during examination, a skin contact device was used consisting of an adhesive ring. Medical ultrasonic couplants were used between the adhesive window and the lens, and water or cane sugar was applied between the adhesive window and the skin.
We obtained images of the same 0.5 × 0.5 mm area at the same skin level individual and face optical sections of the involved skin lesions throughout the skin layers. For each case, three captures (depigmented skin, border of the white macules, adjacent normal-appearing skin) for all patients at the dermoepidermal junction level were investigated.
Histopathology
Tissues were fixed in formalin and embedded in paraffin. After routine processing, 5-mm-thick sections were stained with hematoxylin-eosin for histochemistry analysis. Alternatively, sections were stained with CD4, CD8, CD45RO, CD45RA, and FOXP3 antibodies (BD Biosciences, San Jose, CA, USA).
Description of characteristics under RCM
A score index was used to indicate the progress of vitiligo under RCM. Scoring was based on the following criteria: (1) pigmentation status in the lesional skin. +1 score denoted the existence of remaining pigment [Figure 1a] and −1 score denoted complete loss of pigment [Figure 1b]. (2) Status of the border of vitiligo lesion +1 score denoted indistinct border [Figure 2b] and −1 score denoted clear border [Figure 2a] (3) inflammatory cell infiltration +1 score was assigned if inflammatory cell infiltration was detected at the edge of the lesion [Figure 3] (4) melanocyte regeneration − 1 score was assigned if dendritic melanocytes appeared in the vitiligo lesion [Figure 4]. Grading was as follows: Total score < 1 represented stable stage; ≥ 1 represented active stage; ≥ 2 represented rapid active stage.
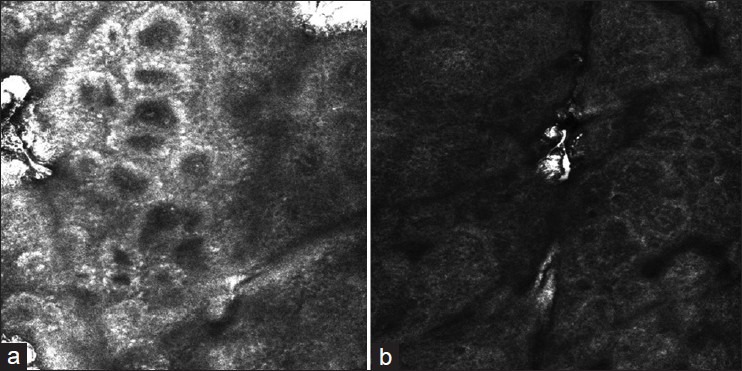
Figure 1.
(a) Reflectance confocal microscopy imaging of lesional skin in vitiligo: The rings lost their integrity and existence of remaining pigment at the dermo-epidermal junction level (b) RCM imaging of lesional skin in vitiligo: Complete loss of pigment at the dermo-epidermal junction level
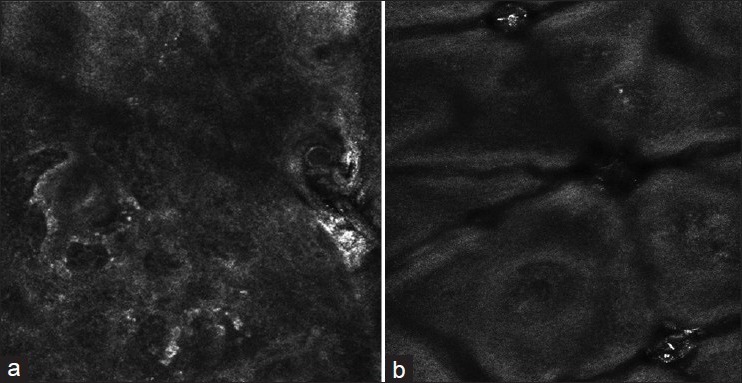
Figure 2.
(a) RCM imaging of lesional skin in vitiligo: Clear border (b) RCM imaging of lesional skin in vitiligo: Unclear border
Figure 3.
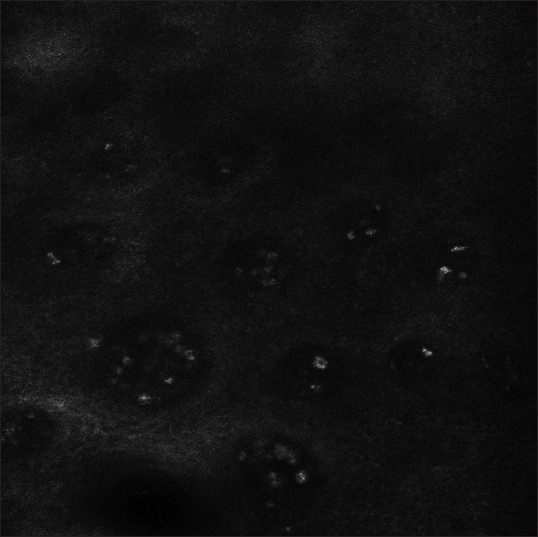
Highly refractile inflammatory cells could be seen at the edge of the lesions
Figure 4.
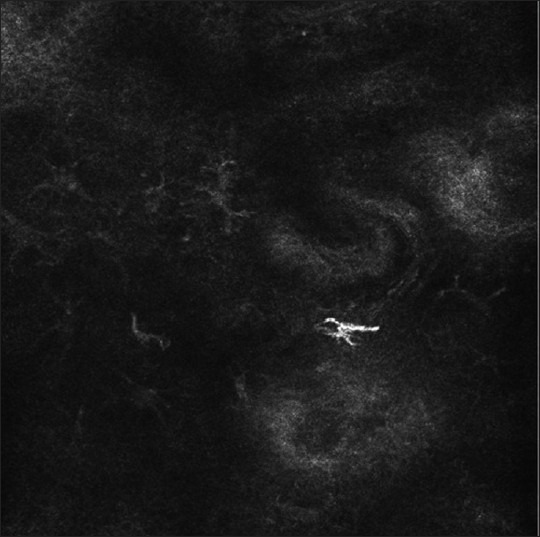
RCM imaging of lesional skin in vitiligo: Dendritic and highly refractile melanocytes can be seen in the lesional area
Results
General results
A total of 125 patients were classified into 3 groups according to the VIDA[1] score: A rapid active stage group (30 patients), a slow active stage group (40 patients), and a stable stage group (55 patients) [Table 1].
Table 1.
The VIDA scoring and RCM analysis comparison of vitiligo staging results

RCM features of vitiligo
In the active stage group, lesional skin showed an apparent loss of melanin under the RCM. Bright dermal papillary rings presented at the dermo-epidermal junction level in normal skin lost their integrity or totally disappeared, and border between vitiligo lesion and normal skin became unclear. Moreover, highly refractile cells that were considered as inflammatory cells could be seen within the papillary dermis at the edge of some of the patient's lesions.
In the stable stage of vitiligo, the RCM demonstrated a complete loss of melanin in lesional skin and a clear border between lesional skin and normal skin. Inflammatory cells could not be found at the edge of vitiliginous skin. Based on the results of RCM analysis, 26 patients were in rapid active stage, 37 patients were in slow active stage, and 62 patients were in stable stage. After RCM examination, 7 more patients were added to the stable group [Table 1].
Histological features of vitiligo
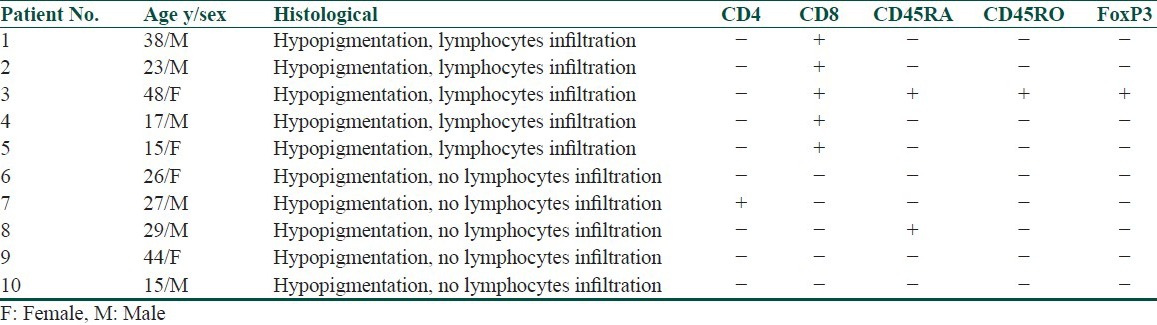
Histochemical examination demonstrated the existence of a large number of lymphocytes infiltration in biopsies, which presented highly refractile cells at the edge of the skin lesions [Figure 5]. Immunohistological analysis showed that those cells were CD8(+), CD4(−), CD45 RA(−), CD4RO(−). Biopsies without highly refractile cells under RCM were confirmed to be absent of lymphocyte infiltration by histochemistry analysis [Figure 6]. Immunohistological results on these biopsies were CD8(−), CD4(−), CD45RA(−), CD45RO(−), FoxP3(−) [Table 2].
Figure 5.
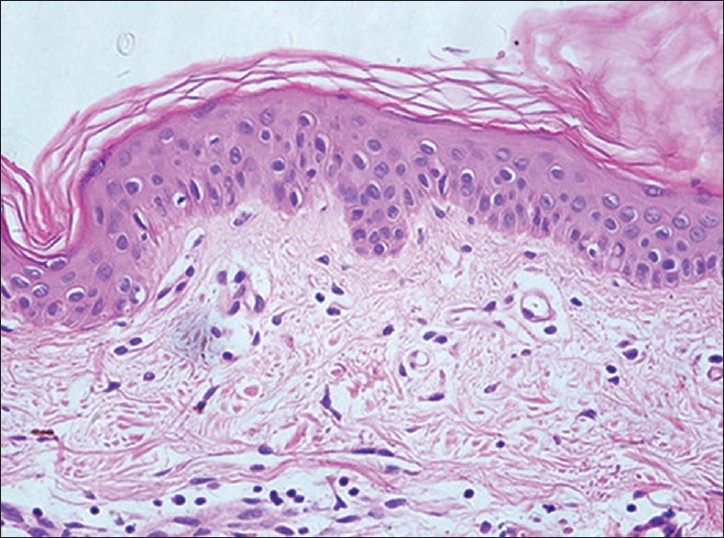
Histological features of vitiligo that on the edge of the skin lesions by RCM can be seen highly refractile inflammatory cells showed (H and E, ×400): Hypopigmentation, lymphocytes infiltration
Figure 6.
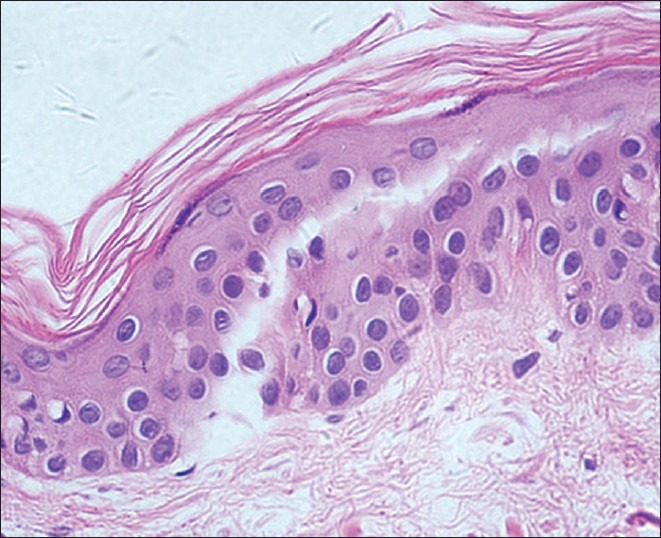
Histological features of vitiligo that on the edge of the skin lesions by RCM can be seen highly refractile inflammatory cells showed (H and E, ×400): Hypopigmentation, no lymphocytic infiltration
Table 2.
Histological features of vitiligo
Discussion
Vitiligo is a depigmentation disorder affecting between 1% to 2% of the general population.[2] The disease is caused by the absence of melanin in the skin and hair follicles. Vitiligo is easy to be diagnosed, but difficult to be treated. It can be divided into two stages clinically: An active stage and a stable stage. As different stages require different treatments: The stable stage is eligible to surgical treatments and phototherapies, while the active stage is likely to respond to steroids, phototherapy/chemotherapy, and immunosuppressants as these drugs are used to arrest the disease progression; therefore, it is important to discriminate these two stages. At present, parameter of vitiligo stage judgment includes VIDA score, Kobner Phenomenon,[1] and Wood's lamp examination.[3] But these parameter have their own limitations and rely mostly on subjective judgments. Therefore, it is necessary to introduce more objective methodologies in order to improve the accuracy of staging. In vivo RCM is a real-time repetitive imaging tool that provides non-invasive images at a nearly histological resolution.[4] The role of RCM in differential diagnosis of melanoma,[5,6,7,8] psoriasis,[9,10] and basal cell carcinoma[11,12,13] has been proven and shown high specificity and sensitivity. In our study, vitiligo staging based on VIDA scoring or RCM analysis was comparable by chi square test. [Table 1, P > 0.05]. Therefore, RCM can be used as a staging tool for vitiliginous lesion. We summarized the features of 125 patients with vitiligo under RCM as follows: Active stage of vitiligo had an apparent loss of melanin in lesional skin, disappearance or lose of integrity of the bright dermal papillary rings normally seen at the dermo-epidermal junction level, unclear border between lesional and normal skin, and highly refractile inflammatory cell infiltration within the papillary dermis at the edge of the part of the patient's lesions. The stable stage of vitiligo had a complete loss of melanin in lesional skin, a clear lesional-normal skin border, and no inflammatory cell infiltration at the edge of vitiligo lesion.
In our study, we demonstrated that highly refractile inflammatory cells seen within the papillary dermis at the edge of a vitiligo lesion could be a good marker to assess the stability. This was consistent with the findings of Lai et al.[14] Histochemical and immunohistochemical examination further confirmed a large number of CD8+ T lymphocytes infiltration at the edge of active stage vitiligo lesions. Since CD8+ T cell-mediated melanocyte destruction is thought to cause the occurrence of vitiligo,[15] indicating the status of lymphocyte infiltration by RCM might be an asset for judging the vitiligo activity. Based on the results of RCM analysis, we re-classified the stage of these 125 patients and found that 7 patients were in the active stage by using VIDA scores displaying stable stage features, among these, 6 patients had segmental vitiligo. We assume that the VIDA scoring is not accurate enough for the assessment of patients with segmental vitiligo. This assumption is supported by the fact that segmental vitiligo usually appears with rapid onset, but with rare further expansion.[16]
In conclusion, RCM is a new imaging tool in dermatology. The ability of RCM to examine lesional skin non-invasively makes it ideal for evaluating the overall responses to therapy. Considering our results, in vivo RCM is an efficient and reliable tool in evaluating vitiligo stage in clinical settings.
What is new?
Reflectance confocal microscopy is a very useful tool helping to determine the stability levels of vitiligo lesions.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Njoo MD, Das PK, Bos JD, Westerhof W. Association of the Kobner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135:407–13. doi: 10.1001/archderm.135.4.407. [DOI] [PubMed] [Google Scholar]
- 2.Huggins RH, Schwartz RA, Janniger C. Vitiligo. Acta Dermatovenerol Alp Panonica Adriat. 2005;14:137–42. [PubMed] [Google Scholar]
- 3.Benzekri L, Gauthier Y, Hamada S, Hassam B. Clinical features and histological findings are potential indicators of activity in lesions of common vitiligo. Br J Dermatol. 2013;168:265–71. doi: 10.1111/bjd.12034. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich M, Lange-Asschenfeldt S, Gonzalez S. Clinical applicability of in vivo reflectance confocal microscopy in dermatology. G Ital Dermatol Venereol. 2012;147:171–8. [PubMed] [Google Scholar]
- 5.Gonzalez S, Gilaberte-Calzada Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci. 2008;30:1–17. doi: 10.1111/j.1468-2494.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 6.Pellacani G, Vinceti M, Bassoli S, Braun R, Gonzalez S, Guitera P, et al. Reflectance confocal microscopy and features of melanocytic lesions: An internet-based study of the reproducibility of terminology. Arch Dermatol. 2009;145:1137–43. doi: 10.1001/archdermatol.2009.228. [DOI] [PubMed] [Google Scholar]
- 7.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216–29. doi: 10.1016/j.jaad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Scope A, Gill M, Benvenuto-Andrade C, Halpern AC, Gonzalez S, Marghoob AA. Correlation of dermoscopy with in vivo reflectance confocal microscopy of streaks in melanocytic lesions. Arch Dermatol. 2007;143:727–34. doi: 10.1001/archderm.143.6.727. [DOI] [PubMed] [Google Scholar]
- 9.González S, Rajadhyaksha M, Rubinstein G, Anderson RR. Characterization of psoriasis in vivo by reflectance confocal microscopy. J Med. 1999;30:337–56. [PubMed] [Google Scholar]
- 10.Ardigo M, Cota C, Berardesca E, González S. Concordance between in vivo reflectance confocal microscopy and histology in the evaluation of plaque psoriasis. J Eur Acad Dermatol Venereol. 2009;23:660–7. doi: 10.1111/j.1468-3083.2009.03134.x. [DOI] [PubMed] [Google Scholar]
- 11.Agero AL, Busam KJ, Benvenuto-Andrade C, Scope A, Gill M, Marghoob AA, et al. Reflectance confocal microscopy of pigmented basal cell carcinoma. J Am Acad Dermatol. 2006;54:638–43. doi: 10.1016/j.jaad.2005.11.1096. [DOI] [PubMed] [Google Scholar]
- 12.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Dendritic cells in pigmented basal cell carcinoma: A relevant finding by reflectance-mode confocal microscopy. Arch Dermatol. 2007;143:883–6. doi: 10.1001/archderm.143.7.883. [DOI] [PubMed] [Google Scholar]
- 13.Ulrich M, Roewert-Huber J, González S, Rius-Diaz F, Stockfleth E, Kanitakis J. Peritumoral clefting in basal cell carcinoma: Correlation of in vivo reflectance confocal microscopy and routine histology. J Cutan Pathol. 2011;38:190–5. doi: 10.1111/j.1600-0560.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- 14.Lai LG, Xu AE. In vivo reflectance confocal microscopy imaging of vitiligo, nevus depigmentosus and nevus anemicus. Skin Res Technol. 2011;17:404–10. doi: 10.1111/j.1600-0846.2011.00521.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Zhou M, Wan Y, Xu A. CD8+ T cells from vitiligo perilesional margins induce autologous melanocyte apoptosis. Mol Med Rep. 2012 doi: 10.3892/mmr.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: The Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25:E1–13. doi: 10.1111/j.1755-148X.2012.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


