Abstract
Bisphosphonates have been shown to reduce mortality in patients with osteoporotic fractures, but the mechanism is unclear. Bisphosphonates have immunomodulatory effects that may influence the development of vascular disease. We sought to determine if bisphosphonate use is associated with a reduced risk of myocardial infarction (MI) in a rheumatoid arthritis (RA) population with high prevalence of bisphosphonate use and vascular disease. Adult patients with RA enrolled in the National Data Bank for Rheumatic Diseases, a longitudinal study of RA patients enrolled continuously from U.S. rheumatology practices between 2003 and 2011, were included in the analysis (n = 19,281). Patients completed questionnaires every 6 months. including questions on medication use, demographic information, clinical information, and health status. MIs were confirmed by a central adjudicator. Among the 5689 patients who were treated with bisphosphonates at some time during the study period, the risk of MI while on bisphosphonate compared to when not on bisphosphonate was 0.56 (95% confidence interval [CI], 0.37–0.86; p < 0.01) after adjustment for multiple confounders. In models including all 19,281 treated and untreated patients, the adjusted risk of first MI was 0.72 (95% CI, 0.54–0.96; p = 0.02) and of all MIs it was 0.72 (95% CI, 0.53–0.97; p = 0.03) in bisphosphonate users compared to nonusers. This finding suggests a potential mechanism for the mortality reduction observed with bisphosphonate medications.
Keywords: Bisphosphonate, Myocardial Infarction, Rheumatoid Arthritis
Introduction
Osteoporosis and the resulting skeletal fractures are a significant worldwide health problem, causing pain, disability, and an increased risk of mortality.(1,2) In addition to the well-recognized excess mortality following vertebral and hip fractures, there are now studies demonstrating that increased mortality occurs with other major and minor fractures.(1,3–6) The causes for the increased mortality are still being debated, and the impact is greater for men than women, and in patients at older ages.(3) Despite these negative consequences of osteoporosis and fractures, most patients who sustain fractures are not offered therapy.(7–9)
A randomized, controlled trial treating patients within 90 days of a hip fracture with an annual intravenous dose of the bisphosphonate, zoledronic acid, or placebo demonstrated a 28% reduction in mortality in the treated group.(10) A post hoc analysis of these data showed that after controlling for baseline risk factors, only 8% of the reduction in mortality could be explained by the reduction in subsequent fracture risk; therefore, 20% of the mortality reduction was due to other factors.(11) Post hoc analyses of two additional trials have since shown that the oral bisphosphonates alendronate and risedronate are associated with similar reductions in mortality when given to patients with hip fractures,(12,13) and a meta-analysis of osteoporosis treatment trials (including bisphosphonates and other medication classes) showed a 10% to 11% relative reduction in mortality.(14) A prospective cohort study found that oral bisphosphonates are associated with reduced mortality in both women and men irrespective of their initial bone mineral density.(15) Although all of these studies support that bisphosphonates reduce mortality in both women and men with osteoporosis, it is not clear which pathways are involved in their mechanism of action.
Rheumatoid arthritis (RA) causes bone loss and osteoporosis due to reduced physical activity, inflammation from the underlying disease, and medications used for treatment.(16–18) RA is also associated with an increased risk of myocardial infarction (MI), thought to be caused at least in part by increased circulating levels of inflammatory cytokines.(19–21) Based on previous epidemiologic studies and known immunologic effects of bisphosphonates, we hypothesized that one mechanism by which bisphosphonates could reduce mortality was by decreasing the risk of MI. In the current study we examined the effect of bisphosphonates on the risk of MI in patients with RA. We selected an RA population because MI occurs with higher frequency in patients with that illness, as does the use of bisphosphonates prescribed for osteoporosis.
Materials and Methods
Design overview
This work was a post hoc analysis of a prospective cohort study using the National Data Bank for Rheumatic Diseases (NDB) longitudinal study of RA outcomes.(22) The NDB utilizes an open cohort design in which patients were enrolled continuously beginning in 1998 and followed until death or study withdrawal. The study database characteristics, including drug assessment methods and validity, completeness of follow-up, and validity of self-reported data has been reported previously.(22–26)
Setting and participants
We included all 19,281 adult patients with a rheumatologist-confirmed diagnosis of RA who participated in the NDB and completed at least two 6-month questionnaires. Participants were volunteers, recruited from the practices of U.S. rheumatologists, who complete extensive mailed or Internet questionnaires about their health at 6-month intervals. Participants were recruited in all 50 states, and were not compensated for their participation.
The study was carried out in compliance with the Helsinki Declaration, and was approved by the Institutional Review Boards of the St. Francis Regional Medical Center, Wichita, KS, the Medical University of South Carolina, and Duke University Medical Center. All patients signed an informed consent.
Interventions
Patients were assessed on a semiannual basis between July 2003 and June 2011. At each assessment we inquired about treatment and events that occurred in the previous 6 months. Treatment data included nonprescription medications, dose, and treatment start and end dates.(22)
Outcomes and follow-up
Case definition of MI
Only MIs that were confirmed by a central adjudication committee were included in this study. As described,(23) possible MIs were identified from study questionnaires, hospitalization records, physician reports, and death records. If primary source documents were not available to adjudicate a potential MI, we contacted the patient’s physician and/or interviewed the patient or family with a structured, preplanned interview designed to address the reported condition. Comparison of patient self-reports with medical records indicates agreement in more than 94% of cases. Review of potential cases was performed by two trained, experienced NDB staff reviewers. This review was followed by an independent physician review. Death records in which MI was recorded as the underlying cause of death must have referred to deaths that occurred within 6 months of the last questionnaire to be included as an MI.
Selection of covariates
To identify possible covariates associated with bisphosphonate prescription, we analyzed potential variables in a multivariable generalized estimating equation (GEE) logistic model that included all study observations. We included sex, age, education categories, household income, body mass index (BMI) categories,(27) prior clinical fractures, the presence of a bone density test, and marital status. In addition, we examined variables present in the last 6 months potentially related to MI risk, including recent MI, hypertension, diabetes, BMI, and smoking. Finally, to assess for long-term risk factors we included “lagged variables” reported to be present prior to the most recent semiannual questionnaire. Lagged variables included Health Assessment Questionnaire (HAQ) score,(28) use of statins, antihypertensives, calcium, and prednisone, and all clinical fractures within the last 5 years. Because statin use was not found to distinguish between bisphosphonate users and non-users in multivariable models, and antihypertensive use was not different between groups, other specific cardiovascular agents were not included in the models.
Statistical analysis
Three models were constructed using different inclusion criteria and modeling strategies in order to assess the sensitivity of the results to different underlying assumptions. Because bisphosphonate treatment is not random and likely relates to underlying MI risk through unmeasured factors such as health behaviors and contact with the healthcare system, our primary model included only patients who had been treated with bisphosphonates at some time during the follow-up period in order to minimize selection bias. Two additional sensitivity models allowed us to examine the effect in ever-treated versus never-treated patients, and to include multiple MI events. In all models, subjects were censored at death or loss to follow-up, and all covariates significantly different at the p < 0.05 level were included.
Model I: treated patients only
Using Cox regression with start time at the first subject observation and bisphosphonate use as a time varying covariate, we estimated the hazard ratio (HR) of a first MI during treatment compared to off treatment, after adjustment for covariates. Thus, subjects contributed “on treatment” follow-up time while taking bisphosphonates, and “off treatment” follow-up time before and/or after their bisphosphonate exposure. The use of a treated-patient model decreases patient heterogeneity, because only patients who receive therapy are evaluated.
Model II: treated patients and untreated patients
We performed Cox regression in all treated and untreated patients beginning with the first study observation, and estimated the effect of bisphosphonates on the risk of the first MI, after adjustment for covariates. The all-patients model allows comparison between treated patients and those who never received treatment.
Model III: treated patients and untreated patients
We used all observations in a GEE analysis with a logit link and robust standard errors to determine the population averaged risk for any MI associated with bisphosphonate therapy, after adjustment for covariates. The GEE model allows evaluation of multiple MIs in an individual patient.
All Cox models satisfied the proportional hazards assumption. The relationship between age and risk of MI was nonlinear. To better analyze the data, age was modeled using a restricted cubic spline with four knots. Data were analyzed using Stata 12.0 (Stata Corporation, College Station, TX, USA). Because the study aims were exploratory, no adjustment for multiple comparison was made.
Covariates were missing in 4% to 6% of observations. Because the data were longitudinal, prior values of fixed characteristics were often available; ie, diabetes reported in a previous observation. In the case of such variables, we replaced the current missing values with the most recent present value. This left between 0.6% and 1.8% of observations with missing covariates. In this instance we used a randomly selected hot-decked replacement or a mean substitution by sex. Because the rate of missingness was very low, we did not use multiple imputation.
Results
Characteristics of patients treated and not treated with bisphosphonates
Eighty-one percent of NDB participants provided at least 6 months of follow-up data and were included in the analysis (n = 19,281). Of these, 5689 used bisphosphonates at some time during the study period: 61.2% used alendronate; 26.2% used risedronate; 12.2% used ibandronate; and 1.4% used etidronate, pamidronate, or zoledronic acid. We combined users of any of the above bisphosphonates into a single “bisphosphonate” user variable. The mean starting year of bisphosphonate therapy was the second half of 2006, and the mean ± SD duration of therapy was 2.5 ± 2.1 (range, 0.5–8.0) years. The mean ± SD duration of follow-up in the study was 4.19 ± 3.0 (range, 0.50–9.0) years. The average dose of bisphosphonate was very similar to the recommended osteoporosis treatment dose for each agent.
As measured at a random observation, patients treated with bisphosphonates differed from those not treated in all demographic and clinical characteristics studied, although some of the differences were small (Table 1). Treated patients were older (67.6 versus 59.5 years of age), had lower household income ($35,000 versus $45,000), were more likely to be female (86.8% versus 75.2%), had a lower BMI (26.5 kg/m2 versus 28.9 kg/m2), had more fractures over the course of the study (26.9% versus 11.7%), and were more likely to be receiving prednisone therapy (43.1% versus 28.5%). With respect to cardiovascular risk factors, diabetes was more prevalent (12.5% versus 10.1%) and statin use more common (21.9% versus 18.3%) in never-users, whereas hypertension (38.2% versus 35.8%) was more common in ever-users. Patients treated with bisphosphonates had more severe RA as evidenced by increased use of prednisone and opioids (26.8% versus 23.8%), and worse functional status as measured by HAQ score (1.2 versus 1.0) and 36-item Short Form Health Survey Physical Component Summary (SF-36 PCS) score (35.3 versus 36.8).
Table 1.
Characteristics of Bisphosphonate Users and Non-Users
| Variable | Patients who ever used bisphosphonates (n = 5891) |
Patients who never used bisphosphonates (n = 13,390) |
|---|---|---|
| Age (years), mean ± SD | 67.6 ± 11.1 | 59.5 ± 13.4 |
| Male sex (%) | 13.2 | 24.8 |
| Education (years) | ||
| 0–8 (%) | 2.6 | 2.5 |
| 8–11 (%) | 7.8 | 6.7 |
| 12 (%) | 37.7 | 32.7 |
| 13–15 (%) | 25.5 | 28.8 |
| ≥16 (%) | 26.5 | 29.3 |
| Median household income ($1000 s) | 35.0 | 45.0 |
| Smoking category | ||
| Never (%) | 57.2 | 52.4 |
| Past (%) | 32.0 | 33.1 |
| Current (%) | 10.8 | 14.5 |
| BMI (kg/m2), mean ± SD | 26.5 ± 5.6 | 28.9 ± 6.5 |
| Hospitalized (%) | 14.2 | 11.2 |
| Fracture during study (%) | 26.9 | 11.7 |
| Comorbidity index (0–9), mean ± SD | 1.9 ± 1.6 | 1.8 ± 1.6 |
| Diabetic (%) | 10.1 | 12.5 |
| Hypertension now (%) | 38.2 | 35.8 |
| Physical component score (SF-36), mean ± SDa | 35.3 ± 10.9 | 36.8 ± 11.3 |
| Mean HAQ (0–3)b | 1.2 ± 0.7 | 1.0 ± 0.7 |
| Prednisone (%) | 43.1 | 28.5 |
| Opioids (%) | 26.8 | 23.8 |
| Ever on vitamin D during observation (%) | 63.4 | 32.6 |
| Ever on calcium during observation (%) | 78.8 | 42.4 |
| Statins (%) | 18.3 | 23.9 |
| Antihypertensives (%) | 48.7 | 42.6 |
Reduction in the risk of MI with bisphosphonate therapy
During follow-up, approximately 1.8% of the cohort experienced a first MI, with 340 events in 19,281 patients. The 5689 patients starting on bisphosphonates at some time during the study period (Model I: treated patients; Table 2) were analyzed in unadjusted and adjusted Cox regressions. The relative hazard of first MI while on bisphosphonates compared to when not on bisphosphonate was 0.53 (95% confidence interval [CI], 0.35–0.81) in the unadjusted model and 0.56 (95% CI, 0.37–0.86) in the adjusted model, with an absolute decrease in the rate of MI from 6.0 MI per 1000 person-years to 2.6 MI per 1000 person years. When all 19,281 treated and untreated patients were considered (Model II; Table 2), the unadjusted relative risk was 0.69 (95% CI, 0.52–0.92) and the adjusted relative risk was 0.72 (95% CI, 0.54–0.96), with an absolute decrease in the rate of MI from 4.3 MI per 1000 person-years to 3.5 MI per 1000 person years. Finally, we used a population averaged GEE model that allowed inclusion of multiple MIs per patient (Model III). As with the other models, bisphosphonate therapy was associated with a protective effect, odds ratio 0.71 (95% CI, 0.53–0.95) for the unadjusted model and odds ratio 0.72 (95% CI, 0.53–0.97) for the adjusted model.
Table 2.
MI Risk Reduction Associated With Bisphosphonate Therapy
| Analyses | Subjects | MIs | Model | RR/OR (95% CI) | p |
|---|---|---|---|---|---|
| Adjusted for age and sex | 5689 | 101 | I: Cox regression (treated patients) | RR 0.53 (0.35–0.81) | 0.004 |
| Full modela | 5689 | 101 | I: Cox regression (treated patients) | RR 0.56 (0.37–0.86) | 0.009 |
| Adjusted for age and sex | 19,281 | 330 | II: Cox regression (all patients) | RR 0.69 (0.52–0.92) | 0.011 |
| Full modela | 19,281 | 330 | II: Cox regression (all patients) | RR 0.72 (0.54–0.96) | 0.025 |
| Adjusted for age and sex | 19,281 | 340 | III: GEE analysis (all patients) | OR 0.71 (0.53–0.95) | 0.020 |
| Full modela | 19,281 | 340 | III: GEE analysis (all patients) | OR 0.72 (0.53–0.97) | 0.030 |
Adjusted for age, sex, education, smoking status, household income, marital status, body mass index, diabetes, hypertension, fracture in the last 6 months, lagged fracture in the last 6 months, fracture in the last 5 years, prior MI, bone density determination in last year. Lagged Health Assessment Questionnaire (HAQ), lagged statin, calcium, and prednisone use.
MI = myocardial infarction; RR = relative risk; OR = odds ratio; CI = confidence interval; GEE = generalized estimating equation.
We were unable to measure the exact time bisphosphonate use was initiated or stopped within a 6-month period, only whether it was used during that period or not. To examine the effect of duration of therapy we assumed that usage within a 6-month period was for the entire 6 months. Under that assumption, using GEE analyses in the fully adjusted model, we found a trend for an association between time on bisphosphonate and MI, OR 0.92 (95% CI, 0.84–1.0; p = 0.053).
In our adjusted Cox analysis for all subjects who had ever received bisphosphonate, we tested for the interaction between previous MI and bisphosphonate effect. Although the test for interaction was not significant (p = 0.12), the hazard ratios (HRs) suggested a possibly greater MI reduction effect in those without a prior MI (HR 0.58) than in those with a prior MI (HR 1.66). There was no significant bisphosphonate by gender interaction (p = 0.93). In sensitivity analyses completed with and without fracture patients included, results were unchanged.
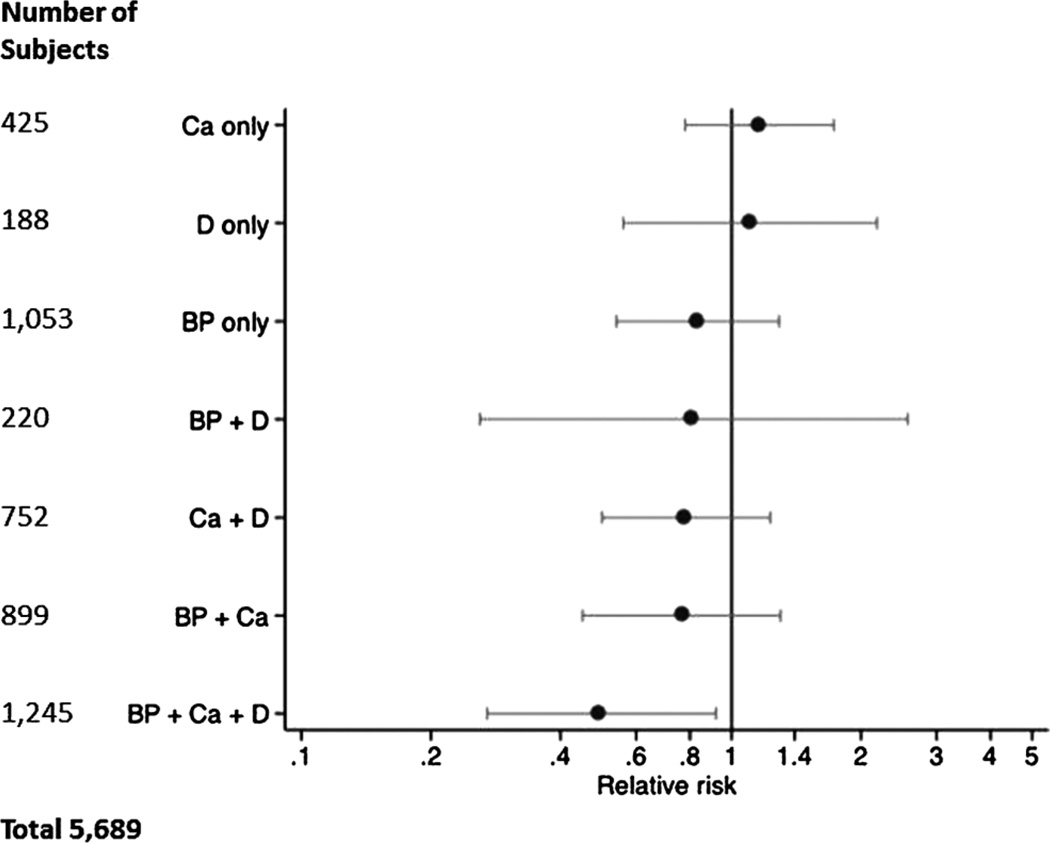
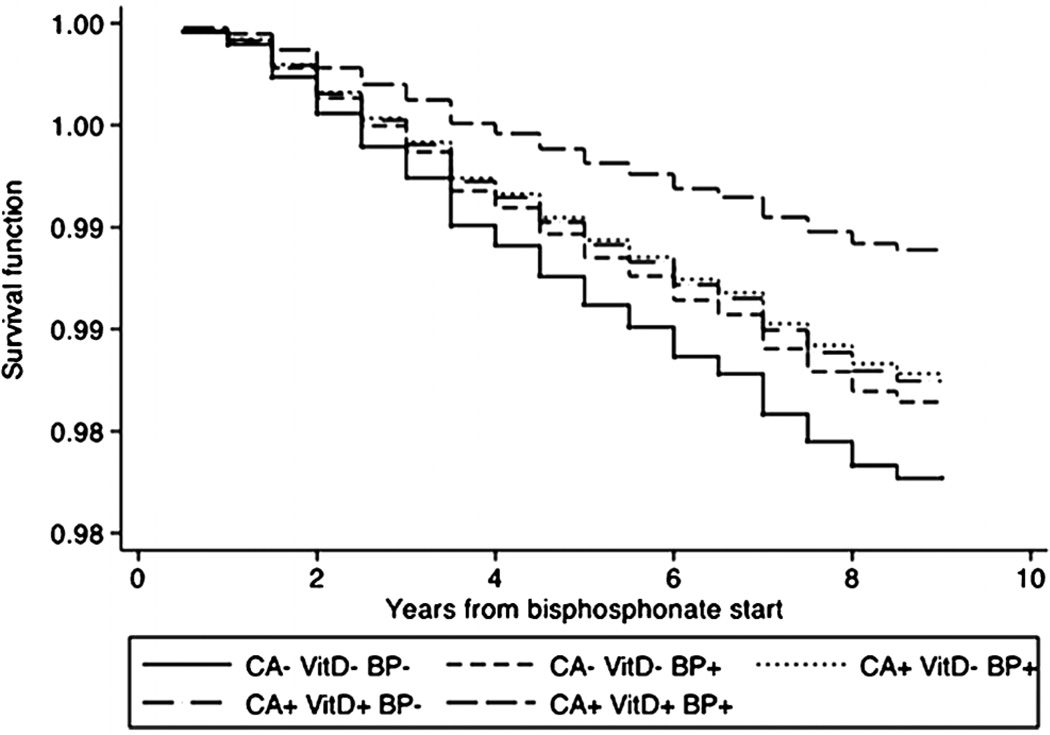
Because calcium and vitamin D have been reported to impact the risk of cardiovascular events,(29,30) we also evaluated bisphosphonate therapy in combination with calcium and vitamin D treatment. The combination of bisphosphonates, calcium, and vitamin D was significantly superior to no treatment, and the effect was consistent across all models. Figure 1 shows the effect of different combinations of bisphosphonate, calcium, and vitamin D therapies on the risk of MI for the Cox model including all patients. Figure 2 displays the proportion of the sample surviving without first MI over time in subjects on bisphosphonates with and without calcium and vitamin D.
Figure 1.
Forest plot of the relative risk of myocardial infarction associated with combinations of calcium, vitamin D, and bisphosphonate therapy (subgroup analyses).
Figure 2.
Survival function for risk of myocardial infarction among groups not treated and variously treated with bisphosphonates.
Discussion
Recent work has demonstrated that treating patients with osteoporotic fractures with bisphosphonates results in both a reduced risk for subsequent fracture and a reduced mortality rate. The reduction in mortality appears after 18 months to 2 years of treatment,(10) and is only partially attributable to a reduction in subsequent fracture.(11) Because previous work suggested a possible impact of bisphosphonates on cardiovascular outcomes,(11,31) we investigated whether the use of bisphosphonates could be protective against MI in a high-risk RA population.
In this study, the risk of MI was substantially reduced among patients with RA who were taking bisphosphonates after adjustment for multiple known cardiovascular risk factors, disease severity indicators, and functional status. The data were consistent in several different sensitivity models, and suggest that a portion of the bisphosphonate effect on decreasing mortality following a hip fracture may be due to a reduction in the rate of MI. Although the absolute risk difference in our study was small given the relatively low incidence of MI in the sample, because cardiovascular disease is one of the most common chronic illnesses in older adults,(32) this is a potentially important finding that, if confirmed in other studies, may have important public health ramifications.
Prior epidemiologic studies and secondary data analyses support a link between bisphosphonate use and reduced cardiovascular disease. A meta-analysis of subjects enrolled in the clinical trials testing risedronate showed a trend toward a lower cardiovascular mortality, driven mainly by a reduction in strokes.(31) Exploratory analyses of a large clinical trial of zoledronic acid suggested a similar incidence, but a lower risk, of death from cardiac arrhythmias in those receiving zoledronic acid, perhaps driven by changes in cardiac ischemia.(11) Nitrogen-containing bisphosphonates were associated with decreased prevalence of cardiovascular calcification in older subjects enrolled in the Multi-Ethnic Study of Atherosclerosis, but more prevalent cardiovascular calcification in younger subjects.(33) More recently, a large case-control study of bisphosphonate users in Denmark reported an inverse dose–response relationship between alendronate use and MI, with a 50% higher increased risk of MI in those with low adherence and a nonsignificant 20% decreased risk in those who were adherent to the drug, with a significant test for trend.(34) A healthy user effect was postulated as one explanation for these findings.
At present, a mechanism for the effect of bisphosphonates on the risk for MI is unknown. Although avidly taken up by bone after administration, bisphosphonates are also deposited in other tissues, including the myocardium and arterial tissue.(35) Therefore, bisphosphonates may have a direct effect on the pathogenesis of MI, rather than indirectly through their impact on bone turnover. In addition, bisphosphonates have systemic effects outside of bone, which may impact both the development of cardiovascular disease and bone turnover. Common pathophysiologic mechanisms linking osteoporosis and cardiovascular disease include suppression of monocyte-macrophage differentiation and function, alterations in serum cytokine levels, and vascular calcium deposition(36–39); bisphosphonates impact several of these areas directly or indirectly. Intravenous, but not oral, bisphosphonates significantly reduce serum low-density lipoprotein in postmenopausal women.(39) Bisphosphonates have an immunomodulatory effect on gamma-delta T cells, which have been shown to mediate cardiac apoptosis.(40) In animal models, bisphosphonates accumulate in arterial tissue and may inhibit macrophage migration and plaque inflammation,(41) although zoledronic acid given immediately before coronary artery ligation in a rat model did not impact macrophage migration or other measures of infarct severity.(42). Finally, bisphosphonates have complex effects on levels of circulating inflammatory cytokines,(43–46), which have been associated with risk of vascular events.
Recent reports suggest a possible association between vitamin D supplements and lower risk of vascular events,(30) and between calcium supplements and a higher risk of vascular events.(29) Because these supplements are frequently but not universally prescribed with bisphosphonates, we performed an exploratory subgroup analysis looking at risk of MI in patients on different combinations of bisphosphonate, calcium, and vitamin D therapy. Overall, the direction of the HRs for most bisphosphonate-treated subgroups favored a protective effect, although the strongest effect in all three models was consistently observed in patients taking bisphosphonate plus both calcium and vitamin D (Table 3, Fig. 1). One possible explanation for our findings is that those taking all three therapies may represent a unique subgroup of highly compliant patients with lower MI risk; however, other recognized markers of compliance including income level and marital status were not associated with MI risk in our models. Alternatively, the limitations in our assessment of calcium and vitamin D supplementation use may have resulted in the pattern observed. To explore the validity of the calcium and vitamin D use self-report, we contacted 109 current bisphosphonate users and noted that, similar to the rate reported in our sample, 60% also used calcium and vitamin D.
Limitations of this cohort study should be noted. The aim of this exploratory study was to examine a potential mechanism of the bisphosphonate mortality benefit, and the findings require confirmation with prospective studies or meta-analysis of randomized trials. Selection bias, in which patients who are prescribed and agree to take bisphosphonates have a different underlying MI risk due to health status, lifestyle, compliance, or other factors, is likely. We attempted to minimize the impact of selection bias in our models by including only bisphosphonatetreated subjects in our main model, and adjusting for the risk of bisphosphonate prescription in our sensitivity models. However, the possibility of unmeasured confounding remains. We chose to study RA patients because the population is enriched for both bisphosphonate prescription and MI, but the generalizability of our findings is uncertain and should be confirmed in other populations. We combined all bisphosphonates together and are unable to distinguish different effects based on route of administration or type, although more than 98% were taking oral agents, primarily alendronate. We did not have measures of bisphosphonate adherence available in the study, although the duration of use was accounted for in our Cox proportional hazards model. Finally, we are not able to determine the time-to-benefit in our population.
Our study also has several strengths. We used three different modeling strategies with different underlying assumptions in different patient groups, and our findings were robust and consistent. Clinical events were adjudicated centrally by study personnel blinded to the study hypothesis. In patients with RA, functional status is a powerful predictor of mortality,(47) and our investigation accounted for this with use of the HAQ score as well as with other variables including use of prednisone and opioid medications. A common bias in observational studies is confounding by indication, which occurs where patients at higher risk for the outcome (MI) are more likely to receive treatment (bisphosphonate) because of a suspected beneficial impact of treatment on MI risk. During the study time period there was no indication in the medical literature that bisphosphonates might reduce the risk of MI, and in fact, some concerns that it might increase risk.(48) Thus, the probability of confounding by indication is low for this study.
In summary, this cohort study in a high-risk population for both osteoporosis and cardiovascular disease found a reduced risk for MI in patients taking bisphosphonates, particularly in combination with calcium and vitamin D supplements. Because both cardiovascular disease and osteoporosis are highly prevalent in older persons, this finding has important clinical implications if confirmed. Further study is warranted to help delineate the mechanism by which bisphosphonates may mitigate cardiovascular risk.
Acknowledgments
The authors report no limitations on access to data or other materials critical to the work being reported.
Disclosures
MBB reports research support from Novartis, Genetech, and Eli Lilly; consultant support from Regeneron; stock in Johnson & Johnson; and Speaker’s Bureau support from Novartis, Genetech, Amgen, and Eli Lilly. CSC-E, CMO, and KWL are co-owners of Biscardia, LLC, and co-inventors in U.S. Provisional Patent Application: “Bisphosphonates and Mortality Reduction” and a second U.S. Provisional Patent relating to bisphosphonates and cardiovascular disease, “Bisphosphonate Compositions and Methods for Treating Heart Failure”. CSC-E is an advisory board member of Amgen, and a consultant for Novartis on osteoporosis-related topics. KWL is an advisory board member for Amgen; a consultant and speaker for Amgen and Novartis; co-inventor in U.S. Patent Application: “Methods for preventing or reducing secondary fractures after hip fracture, ” Number 20050272707, and inventor in U.S. Patent Application: “Medication Kits and Formulations for Preventing, Treating or Reducing Secondary Fractures After Previous Fracture” Number 12532285, and was funded in part through NIH 1P30AG029716-01. KWL and CSC-E are supported by NIA grant 2P30AG028716-06. CMO is a consultant to Amgen and Hoffman LaRoche.
Footnotes
Authors’ roles: Study design: FW, KL, MB, KM, CO, and CCE. Study conduct: FW. Data analysis: FW. Data interpretation: FW, KM, and CCE. Drafting manuscript: CCE, FW, MB. Revising manuscript content: KL, KM, and CO. Approving final version of manuscript: FW, KL, MB, KM, CO, and CCE. FW takes responsibility for the integrity of the data analysis.
References
- 1.Cauley J, Thompson D, Ensrud K, Scott J, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005 Mar;16(Suppl 2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 3.Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Arch Intern Med. 1999;159(11):1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 5.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 6.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture subsequent fracture in men women. JAMA. 2009;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 7.Colon-Emeric C, Lyles KW, Levine DA, House P, Schenck A, Gorospe J. Prevalence and predictors of osteoporosis treatment in nursing home residents with known osteoporosis or recent fracture. Osteoporos Int. 2007;18(4):553–559. doi: 10.1007/s00198-006-0260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar SR, Rowe BH, Folk D, Johnson JA, Holroyd BH, Morrish DW, Maksymowych WP, Steiner IP, Harley CH, Wirzba BJ, Hanley DA, Blitz S, Russell AS. A controlled trial to increase detection and treatment of osteoporosis in older patients with wrist fracture. Ann Int Med. 2004;141(5):366–373. doi: 10.7326/0003-4819-141-5-200409070-00011. [DOI] [PubMed] [Google Scholar]
- 9.Eisman J, Clapham S, Kehoe L. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res. 2004;19(12):1969–1975. doi: 10.1359/JBMR.040905. [DOI] [PubMed] [Google Scholar]
- 10.Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colón-Emeric CS, Mesenbrink P, Lyles KW, Pieper CF, Boonen S, Delmas P, Eriksen EF, Magaziner J. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res. 2010;25(1):91–97. doi: 10.1359/jbmr.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sambrook PN, Cameron ID, Chen JS, March LM, Simpson JM, Cumming RG, Seibel MJ. Oral bisphosphonates are associated with reduced mortality in frail older people: a prospective five-year study. Osteoporos Int. 2011;22(9):2551–2556. doi: 10.1007/s00198-010-1444-6. [DOI] [PubMed] [Google Scholar]
- 13.Beaupre LA, Morrish DW, Hanley DA, Maksymowych WP, Bell NR, Juby AG, Majumdar SR. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22(3):983–991. doi: 10.1007/s00198-010-1411-2. [DOI] [PubMed] [Google Scholar]
- 14.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95(3):1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 15.Center JR, Bliuc D, Nguyen ND, Nguyen TV, Eisman JA. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011;96(4):1006–1014. doi: 10.1210/jc.2010-2730. [DOI] [PubMed] [Google Scholar]
- 16.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County rheumatoid arthritis register. Arthritis Rheum. 2000;43(3):522–530. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Huusko TM, Korpela M, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Threefold increased risk of hip fractures with rheumatoid arthritis in Central Finland. Ann Rheum Dis. 2001;60(5):521–522. doi: 10.1136/ard.60.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vis M, Voskuyl AE, Wolbink GJ, Dijkmans BAC, Lems WF. Bone mineral density in patients with rheumatoid arthritis treated with infliximab. Ann Rheum Dis. 2005;64(2):336–337. doi: 10.1136/ard.2003.017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 20.Sherine EG. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008 Oct;121(10) Suppl 1:S9–S14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Michaud K. The National Data Bank for rheumatic diseases: a multi-registry rheumatic disease data bank. Rheumatology (Oxford) 2011 Jan;50(1):16–24. doi: 10.1093/rheumatology/keq155. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case-control analysis. Arthritis Rheum. 2008;58(9):2612–2621. doi: 10.1002/art.23811. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–1751. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 26.Nadareishvili Z, Michaud K, Hallenbeck JM, Wolfe F. Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: a nested, case-control study. Arthritis Rheum. 2008;59(8):1090–1096. doi: 10.1002/art.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Geneva: World Health Organization; 1995. WHO Expert Committee on Physical Status: the use and interpretation of anthropometry physical status. WHO Technical Report Series: 854. [PubMed] [Google Scholar]
- 28.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9:789–793. [PubMed] [Google Scholar]
- 29.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152(5):315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 31.Steinbuch M, D’Agostino RB, Mandel JS, Gabrielson E, McClung MR, Stemhagen A, Hofman A. Assessment of mortality in patients enrolled in a risedronate clinical trial program: a retrospective cohort study. Regul Toxicol Pharmacol. 2002;35:320–326. doi: 10.1006/rtph.2002.1550. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 33.Elmariah S, Delaney JA, O’Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, Kronmal RA, Halperin JL. Bisphosphonate use and prevalence of valvular and vascular calcification in women: MESA (The Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56(21):1752–1759. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestergaard P. Acute myocardial infarction and atherosclerosis of the coronary arteries in patients treated with drugs against osteoporosis: calcium in the vessels and not the bones? Calcif Tissue Int. 2012;90:22–29. doi: 10.1007/s00223-011-9549-2. [DOI] [PubMed] [Google Scholar]
- 35.Arano Y. Recent advances in 99mTc radiopharmaceuticals. Ann Nuclear Med. 2002;16(2):79–93. doi: 10.1007/BF02993710. [DOI] [PubMed] [Google Scholar]
- 36.Cremers S, Papapoulos S. Pharmacology of bisphosphonates. Bone. 2011;49(1):42–49. doi: 10.1016/j.bone.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Omoigui S. The interleukin-6 inflammation pathway from cholesterol to aging–role of statins, bisphosphonates and plant polyphenols in aging and age-related diseases. Immun Ageing. 2007;4(1):1. doi: 10.1186/1742-4933-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarlane S, Muniyappa R, Shin J, Bahtiyar G, Sowers J. Osteoporosis and cardiovascular disease. Endocrine. 2004;23(1):1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 39.Hamerman D. Osteoporosis atherosclerosis: biological linkages the emergence of dual-purpose therapies. QJM. 2005;98(7):467–484. doi: 10.1093/qjmed/hci077. [DOI] [PubMed] [Google Scholar]
- 40.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of γΔ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102(6):2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 41.Ylitalo R, Monkkonen J, Urtti A, Ylitalo P. Accumulation of bisphosphonates in the aorta and some other tissues of healthy and atherosclerotic rabbits. J Lab Clin Med. 1996;127(2):200–206. doi: 10.1016/s0022-2143(96)90079-7. [DOI] [PubMed] [Google Scholar]
- 42.Hwang H, Hale SL, Leeka J, Kloner RA. Effects of zoledronate in the repair of chronically infarcted rat myocardium. J Cardiovasc Pharmacol. 2010;56(6):604–609. doi: 10.1097/FJC.0b013e3181f71a3a. [DOI] [PubMed] [Google Scholar]
- 43.Cimaz R, Gattorno M, Sormani MP, Falcini F, Zulian F, Lepore L, Bardare M, Chiesa S, Corona F, Dubini A, Lenhardt A, Martini G, Masi L, Bianchi ML. Changes in markers of bone turnover and inflammatory variables during alendronate therapy in pediatric patients with rheumatic diseases. J Rheumatol. 2002;29(8):1786–1792. [PubMed] [Google Scholar]
- 44.Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005;139(1):101–111. doi: 10.1111/j.1365-2249.2005.02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennanen N, Lapinjoki S, Urtti A, Mönkkönen J. Effect of liposomal and free bisphosphonates on the IL-1β, IL-6 and TNFα secretion from RAW 264 cells in vitro. Pharm Res. 1995;12(6):916–922. doi: 10.1023/a:1016281608773. [DOI] [PubMed] [Google Scholar]
- 46.Thiébaud D, Sauty A, Burckhardt P, Leuenberger P, Sitzler L, Green JR, Kandra A, Zieschang J, Ibarra de Palacios P. An in vivo and in vitro study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61(5):386–392. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1530–1542. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein MR. Bisphosphonate therapy vascular calcification. JAMA. 2000;283(11):1424–1425. [PubMed] [Google Scholar]
- 49.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306(6890):1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]