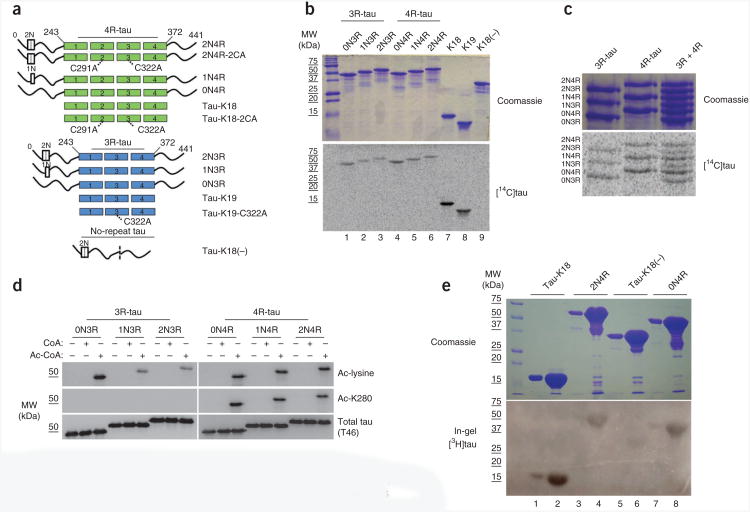
Figure 1.
Tau proteins containing microtubule-binding repeats possess acetyltransferase activity. (a) Schematic representation of all the purified tau proteins used in this study: tau proteins with three or four microtubule-binding repeats (3R- or 4R-tau) and a variable number of N-terminal inserts (0−2N) and tau lacking all microtubule-binding repeats but containing both N-terminal inserts (no-repeat tau). Locations of C291A and C322A mutations in 3R- and 4R-tau are highlighted. (b) SDS-PAGE with Coomassie blue staining (top) of tau proteins incubated with [14C]-labeled acetyl-CoA followed by autoradiography (bottom) to detect acetylated tau proteins. MW, molecular weight. (c) As in b, with 3R-tau or 4R-tau proteins. (d) Immunoblot of 3R- or 4R-tau isoforms incubated in the absence or presence of cold CoA or acetyl-CoA, using antibodies to acetyl-lysine, Ac-K280 and total tau (T46). Ac, acetyl. (e) In-gel acetyltransferase assay (described in Online Methods) with Tau-K18, 0N4R-tau, 2N4R-tau and tau-K18(−), followed by Commassie staining (top) and autoradiography (bottom).