Figure 3.
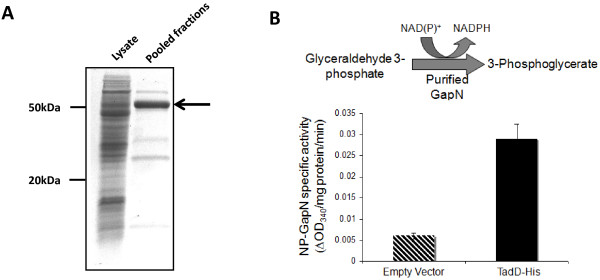
Purified TadD demonstrates NAD(P)+-dependent glyceraldehyde 3-phosphate dehydrogenase (ND-G3PDH) activity. A. SDS-PAGE analysis of purified TadD. A C-terminal hexa-histidine tagged variant of TadD was expressed recombinantly in E. coli from plasmid pDPM21 and purified using nickel affinity chromatography. The arrow marks the recombinant TadD protein. B. Purified TadD reduces NAD(P)+ in the presence of glyceraldehyde 3-phosphate (G3P). The purified histidine-tagged variant of TadD was incubated with G3P and NAD(P)+ and NAD(P)H formation was monitored as an increase in absorbance at 340 nm. Values are expressed as a change in absorbance at 340 nm per mg of protein per minute. We observed a marked increase in specific activity in fractions containing the TadD protein as compared to those from cells harboring the empty vector.
