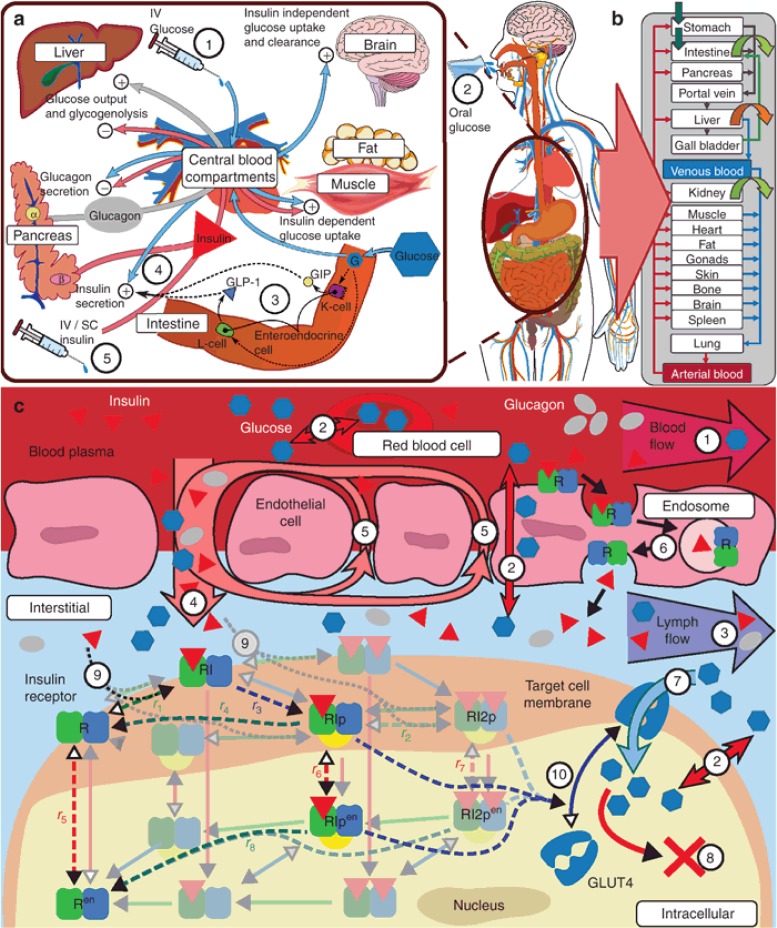
Figure 1.
GIM structure overview. (a) Overview on PD interactions: Glucose has self-regulating effects consisting of suppression of endogenous glucose production, insulin-independent glucose uptake, renal glucose clearance, and stimulation of pancreatic insulin secretion (4), as well as, during episodes of low glucose concentrations, the stimulation of pancreatic glucagon secretion. Insulin, as a glucoregulatory hormone, maintains glucose homeostasis by insulin dependent glucose uptake in muscle and adipose tissue and suppression of endogenous (hepatic) glucose production as well as activation of hepatic glucose uptake. Glucagon, released at low glucose levels, counter-regulates low blood glucose levels, that is, hypoglycemia, through stimulation of hepatic glucose production. Interactions of glucose, insulin, and glucagon vary depending on the type of glucose challenge and pathological conditions. In contrast to intravenous (i.v.) glucose (1), oral glucose (2) additionally triggers the incretin effect (3), which affects insulin secretion (4) via the secretion of gastric hormones (e.g., GLP-1) prior to glucose appearance in the blood. In T1DM insulin secretion (4) is no longer functional, necessitating the infusion of exogenous insulin (5) via the i.v. or subcutaneous (s.c.) route. (b) Organ-level structure of whole-body model: each physiological organ translates to a compartment with several sub-compartments interconnected with convection and diffusion flows (see c). (c) Sub-organ-level and molecular details (1–10): organs are divided into five sub-compartments (blood plasma and cells, endothelial endosomes, interstitial, and intracellular). All compartments are interconnected via passive convection and diffusion flows (1–5) and facilitative transports (7). Distribution of compounds is ultimately dependent on for example, concentration gradients, flow rates, permeability, partition coefficients, transporter properties, and target protein binding properties.24 Both insulin distribution and its glucoregulatory PD effects are influenced or mediated at the molecular level. Bound to insulin receptors (IR) insulin is eliminated from the plasma by trans-endothelial transport (6), and in the interstitial space (9) triggering molecular signaling in target tissues inherently coupling its PK and PD (9, 10). Two receptor models from literature (Quon et al.,25 dashed lines; Koschorreck et al.,26 solid shaded lines) were evaluated. Adapted to the same organism and tissues (human fat, muscle, and liver), both models display similar dynamic properties (data not shown). Therefore, the less complex model by Quon et al. was implemented in the PBPK model, curated and adapted to human physiology (un-shaded receptor-states, see Supplementary Information online). At the molecular level, downstream signaling of the insulin receptor model in fat and muscle triggers translocation of insulin sensitive glucose transporter GLUT4, increasing peripheral glucose uptake (7, 10).