Figure 4. Aptasensor results.
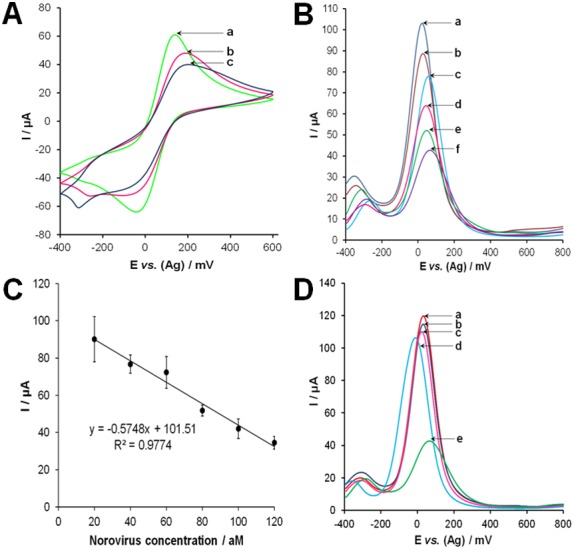
(A) Cyclic voltammograms of the norovirus aptasensor after each immobilization or binding step. The [Fe(CN)6]3−/2− redox couple was monitored for these experiments and cyclic voltammograms were recorded at a scan rate of 100 mV s,–1 where (a) bare SPGE; (b) after self-assembly of the thiolated norovirus specific aptamer; (c) after back-filling with 1 mM 2-mercaptoethanol. (B) Square wave voltammograms obtained using (a) 20 aM, (b) 40 aM, (c) 60 aM, (d) 80 aM, (e) 100 aM, and (f) 120 aM of norovirus in buffer. (C) Calibration plot of current vs. concentration of norovirus. (D) Selectivity experiments performed using (a) buffer alones, (b) 5000 PFU of vesicular stomatitis virus, (c) 5.1 mg mL−1 HSA, (d) 5000 PFU of vaccinia virus (e) 120 aM of norovirus. All experiments were performed in Dulbecco’s phosphate buffered saline after incubation with the developed aptasensor for 1 hr at 25°C. Square wave voltammograms were carried out in the range of −400 to 800 mV with a step potential of 4 mV, amplitude of 5 mV and frequency of 10 Hz. Electrochemical measurements were performed in 25 mM phosphate buffer (pH 7), containing 4 mM K3[Fe(CN)6] and 10 µM hexaamine ruthenium chloride.
