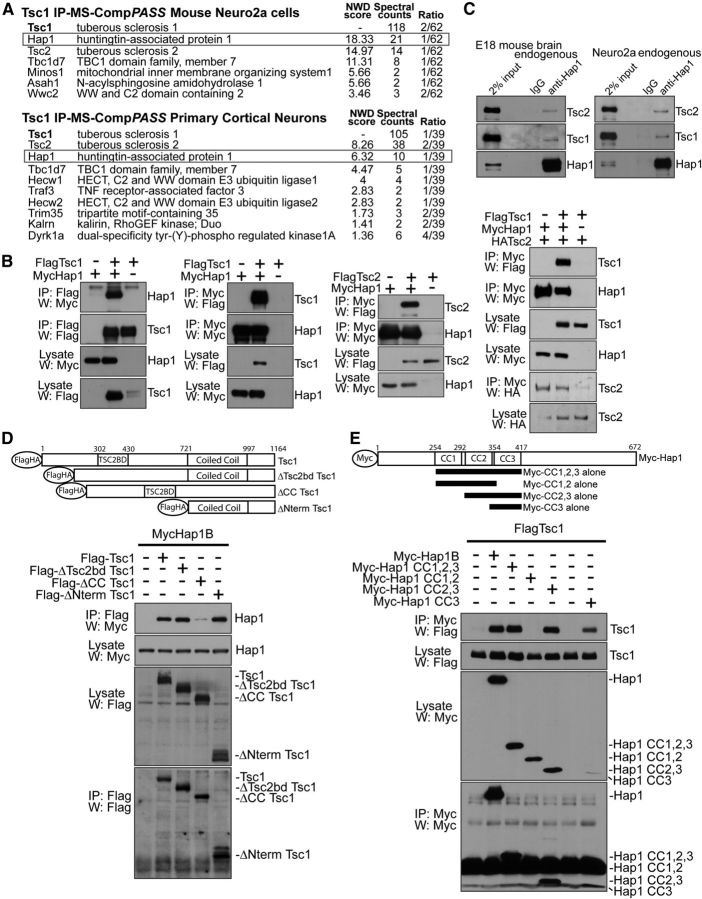
Figure 1.
Identification of Hap1 as a novel Tsc1-interacting protein. A, IP-MS-CompPASS of Tsc1 in neuro2a and cortical neurons. Lysates of stable FLAG-Tsc1-expressing neuro2a or FLAG-Tsc1 lentivirus-infected cortical neurons were FLAG resin-immunoprecipitated. Bait complexes were analyzed by LC MS/MS. Analysis was done in CompPASS against dedicated neuro2a or cortical neuron IP-MS databases. High-confidence interacting proteins had normalized weighted D (NWD) scores >1. Total appearances across unrelated parallel IP-MS bait-runs are shown (“Ratio”). “Spectral counts” represent the number of tryptic peptides detected by the mass spectrometer corresponding to each protein. B, Reciprocal coimmunoprecipitations of Hap1 and Tsc1. First and second panels from left, Lysates of HEK293T transfected with FLAG-Tsc1 and Myc-Hap1B were subjected to immunoprecipitation followed by immunoblotting. Myc-Hap1 interacted with FLAG-Tsc1 and increased FLAG-Tsc1 protein abundance. Third panel from left, Lysates of HEK293T transfected with FLAG-Tsc2 and Myc-Hap1B were subjected to immunoprecipitation followed by immunoblotting. Right, Lysates of HEK293T transfected with FLAG-Tsc1, HA-Tsc2, and Myc-Hap1B were subjected to immunoprecipitation and immunoblotting. HA-Tsc2 was coexpressed to obtain equal levels of Tsc1 because Hap1 expression increases Tsc1 protein abundance. C, Endogenous coimmunoprecipitation of Hap1 and Tsc1. Lysates of E18 mouse brain or neuro2a were subjected to immunoprecipitation followed by immunoblotting. D, Tsc1 deletion mutant coimmunoprecipitation analyses. Lysates of HEK293T transfected with FLAG-Tsc1 deletion mutants and Myc-Hap1B were subjected to immunoprecipitation using FLAG antibody, followed by immunoblotting using Myc antibody. Deletion of Tsc1 coiled-coil domain (ΔCC) but not Tsc2-binding domain (ΔTSC2BD), Tsc1 N terminus (ΔNterm), or the C-terminal 167 amino acids (data not shown), reduced the interaction with Hap1, suggesting that interaction is mediated through Tsc1 coiled-coil domain. E, Hap1 coimmunoprecipitation analyses. Lysates of HEK293T transfected with Myc-Hap1B coiled-coil (CC) fragments and FLAG-Tsc1 were subjected to immunoprecipitation using Myc antibody followed by immunoblotting using FLAG antibody. Hap1 coiled-coil domain 3 (CC3 alone) was sufficient to interact with Tsc1.