Abstract
Background and Aims
Inflorescences are thought to be of enormous taxonomic relevance; however, at the same time they are regarded as being notoriously difficult. This is partly due to the conflicting needs of floristics and evolutionary botany, but partly also due to the complicated and confusing terminology introduced by W. Troll and his school.
Methods
The branching patterns of representatives of the genera Eriocaulon, Syngonanthus and Paepalanthus have been studied in the field and from preserved material by scanning electron microscopy. Branching patterns and formation sequences have been analysed and documented in longitudinal schemes and diagrams. Repetitive units of different levels are detected and related to the body plans of other species of the family.
Key Results
The repetition of very few different branching patterns on different levels of complexity may lead to highly complex inflorescences. However, terms are needed only for patterns; levels may be numbered consecutively. While complex inflorescences are often described as additions or aggregations of units, there is some evidence that complex inflorescences are often the result of fractionation of inflorescence meristems.
Conclusions
Precise descriptions of inflorescences useful for diagnostics and phylogenetics can be much simpler than they often are today. If complex inflorescences are the result of meristem fractionation, intermediate morphotypes cannot be expected. On the other hand, such intermediate morphotypes should occur if a complex inflorescence is formed following an aggregation pathway. Unless the repetitive patterns shown here are not correlated to complementary gene activities the inflorescences are not fully understood.
Keywords: Eriocaulaceae, Paepalanthus, repetitive patterns, meristem fractionation, inflorescence evolution, comparative morphology
INTRODUCTION
Eriocaulaceae can easily be recognized in the field. The identification of genera and species is, however, notoriously difficult. Genera have been and are based exclusively on the morphology of the tiny unisexual flowers (0·3 mm to about 5 mm). Segregates of the large genera Paepalanthus and Syngonanthus are based on characters of the inflorescence structure (Sano, 2004; Parra et al., 2010). The present taxonomic structure is therefore partly more an artificial classification rather than representing a phylogenetic scenario. The morphology of the flowers has been studied by various authors (e.g. Koernicke, 1863; Ruhland, 1903; Stützel, 1984; Giulietti and Hensold, 1991). Recently, Paepalanthus saxicola Koern. and P. syngonanthoides Silveira have been transferred to Syngonanthus based on re-evaluation of their floral morphology (Trovó and Stützel, 2013). Because of the minute size of the flowers, there may be further mistakes in the record. However, knowledge of the floral morphology in the family is rather good. This does not apply to the complex inflorescences of the family.
The flowers are arranged in racemous capitula in all species of Eriocaulaceae. Because of their similarity to Asteraceae, the family has been called the ‘compositae amongst the monocotyledons’ (Eichler, 1875). While the capitula themselves are even more uniform than in Asteraceae, the arrangement of capitula in complex inflorescences is highly variable. The variation is between species and predominantly between higher taxa (section and above). Within species, the patterns are very uniform. This makes inflorescences an interesting target to analyse taxonomic relationships and evolutionary trends within the family.
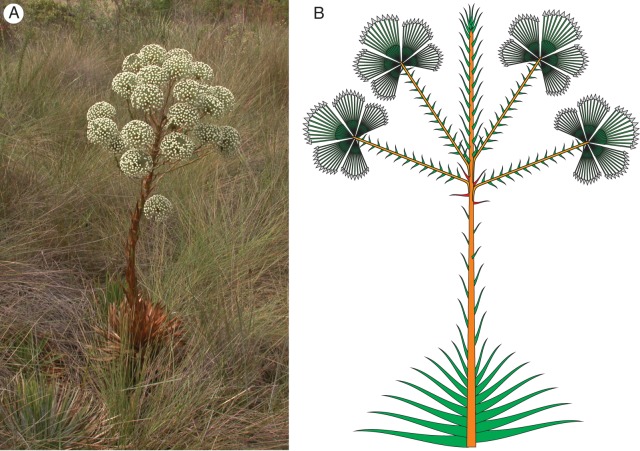
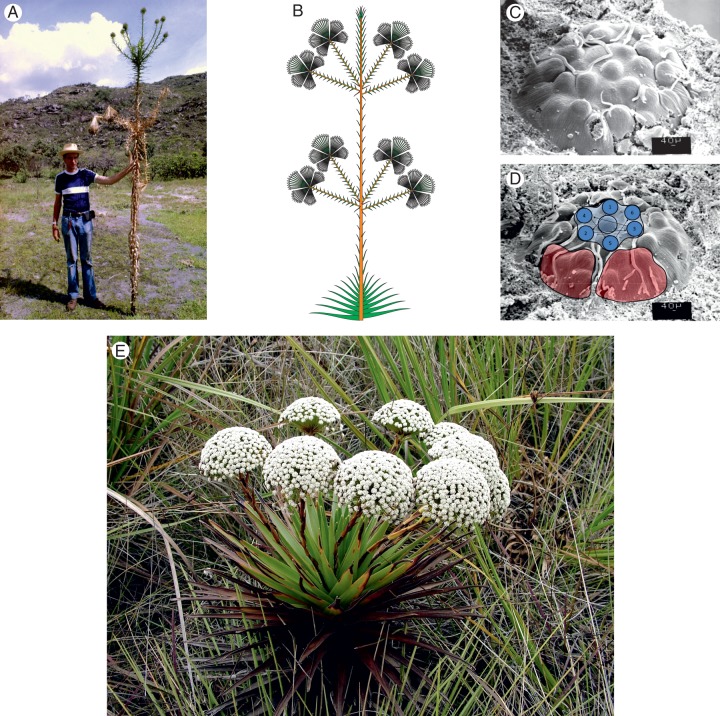
There have been some attempts to analyse the arrangement of the capitula in both the more complex and less complex inflorescences (e.g. Stützel, 1984; Trovó et al., 2010). These studies are, however, either restricted to small groups or are merely theoretical (Stützel, 1984), or restricted to a relatively small group of the family (Trovó et al., 2010). Inflorescence habit has been used to define the genera Actinocephalus (Koern.) Sano (Sano, 2004) (Fig. 3) and Paepalanthus subgen. Platycaulon Koern. (Ruhland, 1903) (Fig. 5D, E). The analyses do not, however, include developmental studies and are not detailed enough to elucidate the evolutionary relationships of these taxa.
Fig. 3.
Actinocephalus polyanthus. (A) Habit; at anthesis the leaves of the hapaxanth plants dry already back and die. (B) Longitudinal scheme of the inflorescence; the umbel-like lateral units have the same structure as the terminal unit in P. erectifolius and are all formed in the axils of a series of consecutive leaves in acropetal sequence. The formation sequence within the umbel-like units has not yet been studied in A. polyanthus. The pattern is assumed to be the same as in in the closely related A. bongardii.
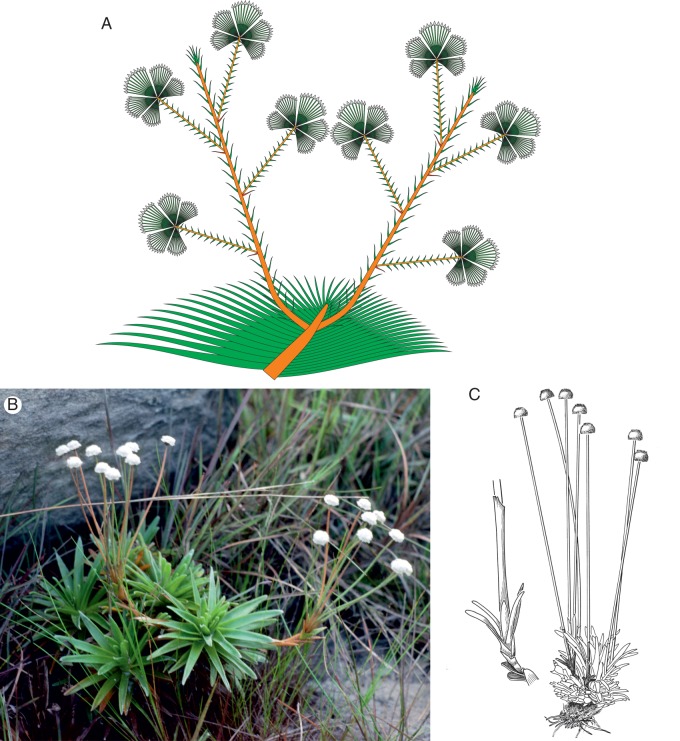
Fig. 5.
(A) Actinocephalus falcifolius, branching scheme. There is a creeping rhizome with a terminal rosette; from this rosette, 1–4 lateral elongate and proliferating branches arise in acropetal sequence. Umbel-shaped aggregations are formed in acropetal sequence. Whether the umbels are formed as in A. polyanthus and A. bongardii or comprise only the terminal botryoid of capitula is still unclear. (B and C) Paepalanthus sect. Aphorocaulon. (B) Paepalanthus incanus has a habit and branching pattern similar to those of Actinocephalus robustus. However, the number of peduncles in each group is much smaller and the individual peduncles are much longer than in A. polyanthus. The units thus do not appear semi-globose to globose, but obconical. (C) Paepalanthus geniculatus has a similar branching scheme. This has a similar branching pattern, but the flowering units are reduced to a single peduncle.
In his comprehensive books, Troll (1964, 1969) made enormous efforts and achieved significant progress towards more comparable descriptions of inflorescences. The initial idea was that it does not matter what inflorescences look like, but what is important is what are the branching pattern and the sequence of formation of the flowers. This approach is helpful in the context of systematics. If the goal is more inflorescence biology similar to floral pollination biology, other aspects might prevail. Here the systematic context is the focus. According to Troll (1964), the first aggregation of flowers leads to a ‘florescence’. There are two basic types, the indeterminate raceme and the determinate raceme called the botryoid. In both of them, the individual flowers may be replaced by cymes which leads to an indeterminate or terminate thyrse. A species has only one of these four types, and repetitions are termed paracladia. As pointed out already by van Stenis (1963) and Claßen-Bockhoff (2000), one of the crucial problems is the delimitation of the inflorescences against the vegetative part. The delimitation is easy if the inflorescence is separated from the latter by a peduncle-like unbranched zone, the so-called inhibition zone. Problems arise if this morphological segregation is absent or if there are more separations within an inflorescence. Trying to overcome such difficulties, Troll (Troll, 1969; Troll and Weberling, 1989) made subsequent additions to his concept and finally it appeared more complex than the inflorescences themselves. The only introduction to the Troll concept in English (Weberling, 1989) suffers from some problematic translations that make some of the logical breaks within the Troll/Weberling approach even worse.
Inflorescences in general are supposed not only to be useful for plant identification, but also to carry an important phylogenetic signal (Troll, 1964, 1969; Weberling, 1981; Troll and Weberling, 1989; Weberling and Troll, 1998). In their treatment of Myrtaceae, Briggs and Johnson (1979) apply a sub-set of the Troll terminology, reject another part as of limited use, but add further terms that received only limited acceptance. Such uniformly repeated sub-units may occur within a taxon several times on different levels of complexity. We therefore try to widen the approach that is implicit in the work by Rua (1969). The goal is to search for repetitive patterns on different levels of complexity. As already demonstrated by Prenner et al. (2009), the number of basic patterns is limited. With respect to developmental genetics, it might be assumed that the same basal pattern is also caused by the same gene. While the basic patterns are most probably homologies, the occurrences of similar combined patterns in different families are frequently analogies (parallelisms). If this is true, the complexity of inflorescences is caused by a variable number and sequence of only a few developmental processes. As the inflorescences within Eriocaulaceae are highly variable and of diagnostic relevance, they seem to be an interesting target group for such an approach.
MATERIALS AND METHODS
Branching patterns have been studied in the field during several joint field trips of the authors. Special care was taken to include only individuals which did not show any damage of a terminal or lateral apex, e.g. by insects or pasture. As the seeds in Eriocaulaceae are often not shed singly, but in entire fruits with two or three seeds or even in entire capitula, it was also important not to mix up groups of individuals with a branched plant. Therefore, rosettes have been analysed directly in the field to detect the position and number of renewals. In rosulate species, the phyllotaxis and position of the capitula were analysed by making transverse sections through entire rosettes directly above the leaf insertion. These cuts were photographed and the photographs were used as the basis for drawings using CorelDRAW® to analyse the branching patterns. In species with elongated stems such as Actinocephalus, longitudinal schemes have been made directly in the field.
From the broader analysis that has been done in the field, 14 species belonging to Eriocaulon, Syngonanthus, Paepalanthus and Actinocephalus (a segregate of Paepalanthus sensu lato) have been selected to demonstrate the main traits within the family. Most emphasis was put on Paepalanthus (including Actinocephalus), as the intrageneric structure is in parts still badly resolved, and we assume and partly demonstrate that it could be resolved better using inflorescence morphology. We start with those species that show rosettes terminated by an inflorescence and proceed to those showing different levels of truncations. Proliferating rosettes with axillary capitula are thus dealt with at the end. This sequence is also suggested by the fact that gynoecium morphology indicates that these are the most derived types within Paepalanthus, and in the other genera this type seems not to occur.
Capitulum or head is used here in the same sense as in Asteraceae. The capitula generally terminate a single distinct and long internode that is called a peduncle here as this term is generally used in Eriocaulaceae. As it is leafless it could be also and more precisely called a scape. At the base, this scape is surrounded by a closed tubular sheath that is formed by one single leaf. This sheath may be truncate transverse or oblique. In the latter case, the tip indicates the position of the leaf in the phyllotactic sequence and can be used to detect branching patterns. The arrangement of capitula formed from one rosette in one flush or flowering period is called an inflorescence. The comparison between species shows that parts that are formed in one species in one flush are flowering in a closely related species in several subsequent seasons. In this case, we use the term ‘proliferating inflorescence’ and indicate for how long this type of continuous inflorescence formation will go on.
‘Pherophyll’ is used here for a leaf bearing a lateral shoot in its axil. The widely used term ‘subtending leaf’ is somewhat ambiguous as it is also used for leaves inserted on the same axis as the subtended structure, e.g. for the spathe in Araceae subtending the spadix.
For species with composed capitula (Paepalanthus subgen. Platycaulon), central parts of rosettes of plants that seemed to be close to flowering were fixed in formaldehyde, acetic acid and ethanol (FAE) for subsequent study by scanning electron microscopy (SEM). These stages were too young to be detected without a dissecting microscope and so this was concluded from other individuals in the same population already showing young budding inflorescences. In the same way, very young stages of the umbel-shaped stands of capitula in Actinocephalus were collected. Critical point drying was done after dehydrating using formaldehyde dimethyl acetate (FDA) (Gerstberger and Leins, 1978). While the early stages of the development can be documented using this technique, older stages develop a pilose receptacle for the capitula as well as for the stands of capitula. SEM pictures show reasonable results only if all these hairs are removed, which is frequently not possible. Such stages are therefore documented by line drawings done from fixed material as the hairs are more or less translucent in a fully hydrated stage. As the developmental stage cannot be recognized unless the entire dissection is completed, the number of useful samples was, however, limited in some species.
RESULTS
Eriocaulon modestum Kunth
Eriocaulon modestum (Fig. 1A) is a small rosulate herb occurring along the Brazilian coast and also scattered in some mountain regions. Growing terrestrially, its leaves are about 5 cm long and the peduncles about 15–20 cm high. Growing submerged, the leaves may be up to about 30 cm in length and the peduncles become about 10–20 cm longer than the depth of the water. Capitula have also been found flowering submerged. However, this might also be an effect of a rapid rise of the water level after rain in the mountains. The species usually has only few capitula per rosette (1–5), and in rosettes with only a single capitulum it is relatively easy to detect that the capitulum terminates the axis of the rosette (Fig. 1B). The sheath takes the position that would be expected in the phyllotactic spiral of the rosette leaves. As the sheath is obliquely truncated in this species, the position of the sheath can easily be detected from the position of the sheath tip. There may be lateral branches in the leaf axils directly preceding the terminal capitulum (Fig. 1C). These may terminate in a capitulum after forming several vegetative leaves. The uppermost branches are stronger but form fewer leaves before they terminate in a capitulum. The formation of additional lateral branches and capitula on the same axis is thus in a basipetal or centrifugal sequence. The most basal lateral branches serve as renewals for the next flowering season. Innovation thus takes place more or less from the centre of the rosette from the last leaf or the last few leaves preceding the sheath.
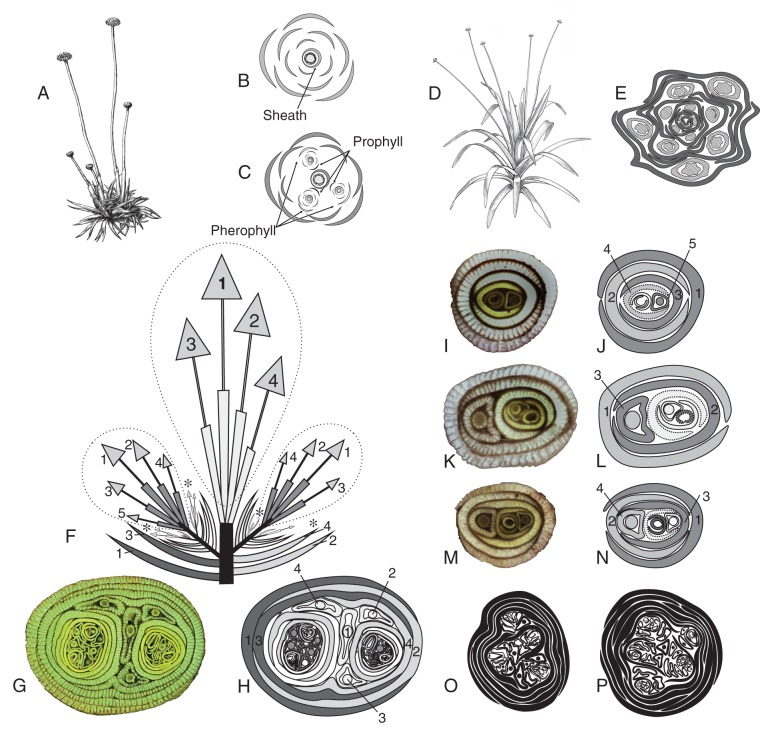
Fig. 1.
(A–C) Eriocaulon modestum. (A) A plant with five peduncles arising from two joint rosettes. (B) Scheme of a transversal cut through a rosette with only a single peduncle showing that the latter is terminal to the rosette. (C) Lateral shoots also terminating in a peduncle may occur in the axils of leaves directly preceding the terminal peduncle. The number of vegetative leaves is variable and highest in the basal-most lateral shoot. (D and E) Paepalanthus catharinae. (D) Habitus. (E) Transversal section through a rosette at flowering time; peduncles are solitary axillary and are formed in a continuous acropetal sequence. (F–P) Eriocaulon megapotamicum. (F) Longitudinal scheme of a flowering plant with 18 capitula. (G) Photograph of a transversal section through a rosette. (H) The same rosette, with leaves and capitula inserted on the main axis numbered in the sequence of formation, capitulum no. 1 terminating the main axis. (I, K, M) Transversal cut through small rosettes at the level of leaf insertion. (J, L, N) Scheme of the same rosettes to illustrate the branching pattern. Leaves on the relative main axis numbered consecutively; prophylls with dotted margin. (O, P) Transversal sections through the base of larger rosettes. In larger plants, the phyllotaxis changes from distichous to alternate when the reproductive phase starts. The distichous leaves are generally partly or entirely rotten at that time and are thus missing from the schemes. Due to the lack of prophylls for the flowering units in the axils of the uppermost leaf axils, it is difficult to distinguish the peduncles of the terminal unit from those in the uppermost axils.
Eriocaulon megapotamicum Malme
Eriocaulon megapotamicum is much larger than E. modestum. The leaves are up to 50 cm long, more or less erect and up to 10 cm wide. The peduncles may reach about 1 m in length. The phyllotaxis changes from distichous in the vegetative phase to spiral in the reproductive phase. Renewals are again distichous. However, this change in phyllotaxis is difficult to see in herbarium specimens as vegetative material is rarely collected. Very weak plants may form their single capitulum without changing to the spiral phyllotaxis, and this facilitates the analysis of such plants.
Generally the position of the sheath apex can be detected easily in the transverse section as the sheath is thicker in the median part. If not, the orientation can be seen from the oblique truncation of the sheath apex. Leaf no. 4 (Fig. 1j, dotted) is the pherophyll of an axillary shoot with two leaves (bright grey). The phyllotactic pattern would also allow the first leaf of the lateral shoot to be regarded as leaf no. 5 of the main shoot. In this case, the position of the sheath would, however, be inverse to what would be expected according to phyllotactic rules. The prophyll has to be with its dorsal side towards the main axis, and this is only the case if the interpretation given here is adopted. In monocot inflorescences, the adorsed position of the prophyll may also be slightly dislocated to a more lateral position, but it is never shifted to the abaxial side.
In Fig. 1K, L the pattern is basically the same. A slight shift towards a spiral phyllotaxis can be seen, and the first-order lateral axis is also terminated by a capitulum with a sheath. The second leaf of the first-order lateral axis (dotted) serves as a pherophyll for a second-order lateral axis represented only by the nearly adorsed prophyll. The most important difference between Fig. 1N and L is that the prophyll of the first-order lateral axis serves directly as the pherophyll of a second-order lateral axis.
In Fig. 1F–H, a much more complicated individual and the analysis of the ramification pattern are shown in a longitudinal (Fig. 1F) and transversal scheme (Fig. 1H) and a photograph of a transversal section of the rosette (Fig. 1G). A total of 18 capitula are arranged in six groups of 1–5 capitula. There is an obvious difference in age between the different groups, which can be detected from the diameter of the peduncles, the length of the peduncles, the diameter and flowering sequence of the capitula, and – to some extent – from the colour of the leaves. As the leaf growth is exclusively basiblast, younger leaves are cut nearer to the apex than older ones and thus appear more intensely yellowish green. The most obvious indicator is, however, the relative position within the rosette. Leaf nos 1–4 are obviously inserted on the main axis. Leaf no. 3 and no. 4 serve as pherophylls for first-order lateral shoots. The main shoot is terminated by a group of four capitula, of which the one in the middle of the rosette has the longest peduncle and therefore seems to be the one terminating the main axis. There are, however, no pherophylls for the other capitula. The position of the sheath is not detectable on the transverse section as the rather wide sheaths are folded irregularly due to lack of space, and the thickness of the sheath is about the same along the entire circumference (in contrast to the small individuals shown in Fig. 1I–N). On the intact plant, the sheaths appear to be positioned towards the centre of the group instead of towards the periphery of the rosette, indicating that the sheaths are the prophylls of the peduncles. The same pattern is repeated in both first-order lateral shoots. The lateral shoot on the left is terminated by a group of five, the one on the right by a group of four peduncles, each peduncle with a surrounding sheath. Again, in the axils of the two uppermost non-sheathing vegetative leaves, continuations are placed on either side (Fig. 1F, marked with asterisks), each starting with a rather perfectly adorsed prophyll. Three of these four shoots terminate in a group of two or in a single capitulum; the fourth seems to be still vegetative.
It is important to note that all capitula of the plant illustrated in Fig. 1F–H are flowering in the same season. The analysis was performed in December; the flowering period may extend from late October to February. Within this period, the pattern described above may be repeated several times. After the end of the flowering period during winter (March to September), continuations in similar positions produce a much larger number of leaves in clearly distichous order. It has to be noted that even the plant in Fig. 1F–H is a smaller one, indicated by the distichous arrangement of the vegetative leaves. Larger individuals shift to an alternate phyllotaxis before they develop inflorescences and produce far more peduncles (Fig. 1O, P). In such individuals, it is much more difficult to analyse the ramification pattern.
Syngonanthus caulescens (Bong.) Ruhl.
Syngonanthus caulescens is a widespread and highly variable species. At high altitudes in mountain areas, on poor soils and freely exposed, the species may have an acaulescent habit with only few capitula. Under favourable conditions, however, it develops the typical umbellate habit (Fig. 2D). In younger stages it can be seen that the development also starts from the top of the inflorescence and proceeds in basipetal sequence (Fig. 5E). The central capitulum is the oldest one. It seems to be the first to be formed in the sequence of capitula and it is the first to start blooming. The other capitula all have their sheath in an adaxial position, which indicates that the sheath is the prophyll of the lateral capitula in a composed inflorescence that is terminated by a sheathed peduncle with its capitulum. Further capitula may be formed in a basipetal sequence (Fig. 2F). However, in inflorescences, there is no fixed number of elements similar to most flowers. Therefore, Fig. 2E cannot be regarded as a younger stage of Fig, 2F. Both may have different numbers of capitula ab initio. The structure is thus essentially the same as for the groups of capitula in E. megapotamicum. However, the number of capitula is significantly higher than in this species. The spiral arrangement of the lateral capitula is best indicated by the arrangement in clockwise and counterclockwise parasticha.
Fig. 2.
(A and B) Paepalanthus erectifolius. (A) Habit; most parts of the basal rosette are already rotten when the plant is flowering. (B) The umbel-shaped stand of capitula is composed of several units in the axils of well-developed pherophylls; the units are formed in a basipetal sequence; within these units, pherophylls are lacking. (C) Syngonanthus chrysanthus. The cushion-like groups of rosettes may originate from different seeds, but can also be lateral branches emerging from the lower side of a rosette of the previous year; a rosette may bear a single or up to about 150 capitula. (D–F) Syngonanthus caulescens. (D) A caulescent stem carrying an umbel of capitula. (E) Young stage of a stand of capitula; the arrangement in parasticha can be seen; pherophylls for the individual capitula (including their sheath) are lacking; the adorsed position of the sheath indicates that it represents the prophyll. (F) Slightly older stage of a similar inflorescence.
After anthesis at about seed ripening of the terminal unit, in the axils of the uppermost leaves lateral inflorescences of the same structure may be formed, which may be preceded by one to several leaves and even folious stems. If fruiting erect stems of S. caulescens are washed down, lateral inflorescences may be formed in the axil of any leaf of the stem. In herbarium specimens this is difficult to be seen, but it is indicated frequently by the lateral inflorescences all being directed to one side. Usually, S. caulescens seems to be annual or at least short-lived. Under favourable conditions, iterations (renewals) may occur from the base of the caulescent stems after seed dispersal and usually after all or most parts of the terminal umbel have dried back. However, it may be difficult to distinguish basal renewals from plantlets originating from different seeds even in the field. In herbarium specimens, this is mostly impossible.
Syngonanthus chrysanthus (Bong.) Ruhland
Syngonanthus chrysanthus has an inflorescence structure that is very similar to that of S. caulescens, but never develops an elongated stem (Fig. 2C). Weak plants have a rosette diameter of <3 cm and only a single capitulum. In strong plants, the rosette diameter may be up to 20 cm and the number of capitula may exceed 150, all forming a single botryoid of capitula. In many places, the species appears to be hapaxanth. Under favourable conditions, one or several renewals may be formed at the base of the rosette. If there is only one renewal, the peduncles persisting sometimes for several months on the plant are all shifted to one side of the new rosette. If several renewals are formed, this may lead to a circular arrangement of the new rosettes. The remains of the previous rosette in the centre may still be present or can disintegrate completely before the lateral rosettes start flowering. Theoretically this growth mode may leads to cushion-like arrangements of inflorescences, and under greenhouse conditions this in fact happens. In the field, cushions seem to be rare in this species, but circular or garland-like arrangements of daughter rosettes of one individual have been observed.
Paepalanthus erectifolius Silveira
The inflorescence of P. erectifolius looks umbel-like; it is, however, composed of several units of the type described for S. caulescens as the terminal unit (Fig. 2A, B). One of these units terminates the long caulescent stem; the others are placed in the axils of several bracts directly preceding the terminal unit. As in S. caulescens, within all these units no pherophylls for the individual peduncles are formed. One of the peduncles in the centre of each unit is longer than the others, and this one is thought to terminate the unit; developmental studies in this species are, however, lacking. The plant growth starts with a rosette of relatively long leaves. When the inflorescence formation starts, the rosette leaves dry back, and they are usually rotten when the plant is finally at anthesis. The leaves on the erect stem are much smaller than the rosette leaves (only half to one-third of the length of the rosette leaves). Rosette leaves are usually lacking on herbarium specimens, and it is unclear how long the process from germination to blooming takes. Generally there seems to be no renewal from the base or from the inflorescence; the species is hapaxanth.
In case the developing inflorescence is damaged or removed, e.g. due to pasture, several lateral inflorescences are formed, each repeating the pattern of the original terminal inflorescence. Such plants are smaller and have many more capitula than undamaged plants. As they tend to fit better on a herbarium sheath, they are found frequently as herbarium specimens.
Actinocephalus polyanthus (Bong.) Sano
The inflorescences of this species are composed of up to about 20 lateral branches formed in a series of consecutive leaves on a stem up to about 1 m high (Fig. 3A, B). The elongate stem arises from the centre of a big rosette which seems to grow vegetatively for several years until it finally changes to the flowering stage by producing an elongate flowering stem from the apical meristem of the rosette. Each of the lateral branches terminates in an umbel of >100 capitula, each of them bearing about 100–150 flowers. The main axis remains sterile; the species is hapaxanth and dies after flowering. The initiation of the lateral flowering units starts from the rosette prior to the elongation of the main axis. This leads to concaulescent shifts of the lateral branches nearly up to the lower side of the next leaf. During the intercalary growth, the pherophylls of lateral umbel-like groups of capitula are often ruptured longitudinally because of their oblique insertion.
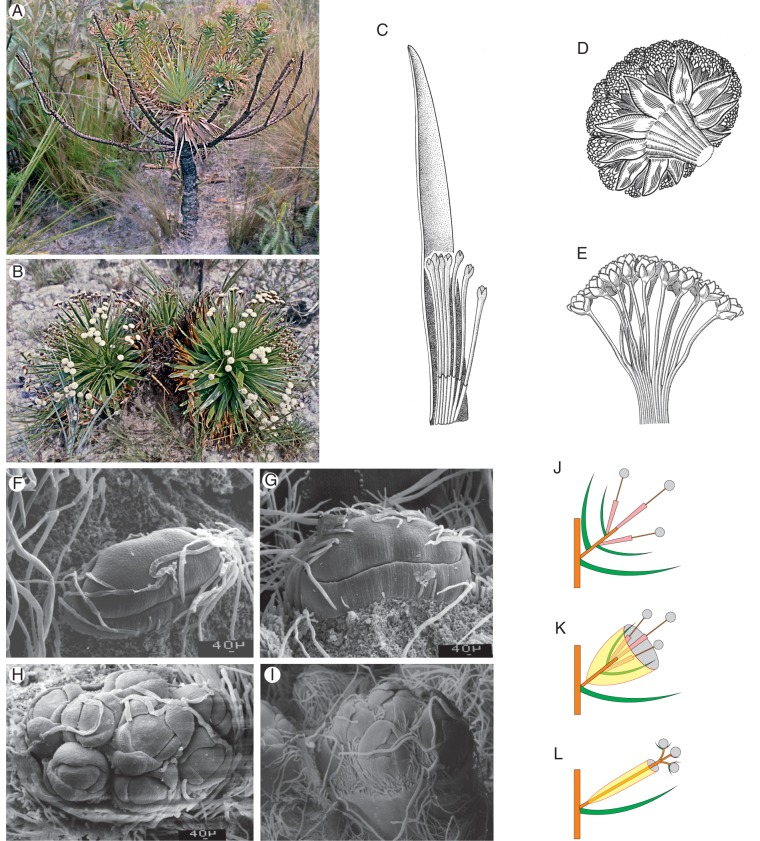
Actinocephalus bongardii (A. St.-Hil.) Sano
Like A. polyanthus, the species forms a large plurienne rosette (Fig. 4A, B). The shift to the reproductive phase seems to take much longer than in the previous species, and at anthesis the basal rosette is usually no longer present. The rosette is therefore lacking in herbarium specimens and is also not mentioned in the original description of the species. The number of the lateral units terminated with umbel-like stands of capitula is only 3–5, and thus generally much lower than in A. polyanthus. As metatopies are lacking, the intercalary growth seems to be largely finished when the flowering lateral braches are formed. In contrast to A. polyanthus, the main axis of the plant generally goes on growing vegetatively for a longer period. After a while (it is unknown whether this is a year or only another wet season of the same year), a second group of lateral umbels may be formed. Each group of umbels arises from consecutive leaves on the erect stem. Subsequent groups are separated from each other by a larger number (20 up to >50) of sterile leaves. Up to three groups of umbels representing different flowering periods have been reported before the plants finally seem to die.
Fig. 4.
(A–D) Actinocephalus bongardii. (A) T. Stützel with a plant at anthesis, the basal rosette has deteriorated as in P. erectifolius; umbel-like units are formed laterally to the main axis. In contrast to P. polyanthus, after a phase of vegetative growth, a set of new lateral units can be formed. (B) Longitudinal scheme. (C and D) Development of the umbel-shaped units, (C) without and (D) with annotation of the subunits and sequence of formation within the terminal unit. (E) Actinocephalus robustus forms a monopodial erect rosette growing for many years; umbel-shaped lateral inflorescences are formed in the leaf axils of 5–10 subsequent leaves. The species lacks vegetative branching (photo: Livia Echternacht).
For A. bongardii it was possible to analyse the ramification pattern within the umbel-like arrangements. The primordia in Fig. 4C, D will form entire capitula including the sheath. Young umbels show a terminal unit representing a botryoid of capitula with one terminal capitulum (marked) and several lateral capitula in a basipetal (centrifugal) sequence. The terminal unit is surrounded by several similar units; two of them are marked in Fig. 4D. The lateral units are, however, deformed due to the limited space, so that the formation pattern is difficult to detect. The ‘umbel’ in A. bongardii thus represents the same structure as the entire terminal inflorescence in P. erectifolius.
Other species of Actinocephalus
In Actinocephalus robustus (Silveira) Sano (Fig. 4E) and A. brachypus (Bong.) Sano there is no distinction between a basal (or juvenile) rosette and an elongated stem at flowering, but the rosette seems to proliferate for a long time and forms lateral umbel-shaped inflorescences for many years. The rosette shows no basal renewals, and a little monopodial trunk is formed until the plant dies after an unknown number of years. In Actinocephalus falcifolius (Koern.) Sano, a creeping rhizome is formed bearing a terminal rosette of relatively small leaves (about 5 cm long and 5 mm wide). Reproductive shoots are in the axils of the rosette leaves. Each reproductive shoot is indeterminate and bears lateral umbels of capitula. These umbels are formed in acropetal sequence. Paepalantus falcifolius is the only species for which this pattern is known as yet.
Paepalanthus sect. Aphorocaulon Ruhl
Paepalantus incanus Koern. (Fig. 5B) looks at first sight similar to a minute Actinocephalus robustus. The lateral branches originating from the rosette form only a single botryoid of capitula. The number of capitula is small, and the peduncles are rather long so that an obconical shape of the lateral units results. Because of limited material of this rare species, it is not yet clear if the rosettes can branch vegetatively or if the group of rosettes in Fig. 5B represents several individuals.
Paepalanthus polygonus Ruhl. and Paepalanthus eriophaeus Ruhl
Paepalanthus polygonus is outstanding in the family because of its arborescent habit (Fig. 6A). The leaves on the vegetative vertical main axis are about twice as big as those on the flowering lateral branches. The lateral braches seem to branch dichotomously, but there are sometimes also trifurcations, which indicates that the branching is generally axillary. Paepalanthus eriophaeus (Fig. 6B) lacks a sterile main shoot and shows a branching rosette without elongated stem. In this way, cushions of a few to several rosettes are formed. Both species are characterized by a series of peduncles inserted together in the axil of the same pherophyll (Fig. 6C). Each peduncle is supplied with its own closed sheath, and a series of about 20 of such fertile leaves is formed in acropetal sequence each flowering season. Both species are obviously perennial. Fertile and sterile leaves are identical in size and shape. The remains of many flowering periods as well as traits of fires indicate that the species is long-living. The densely packed leaf bases on the stem seem to serve for fire protection and do not get burned.
Fig. 6.
Branching patterns in closely related groups of Paepalanthus. (A) Paepalanthus polygonus has a unique arborescent habit. (B) Paepalanthus eriophaeus forms cushions of from three to more than six rosettes; in this specimen at the time of anthesis of one flush of capitula, a distinct second, still budding flush appears more apically. (C) Paepalanthus polygonus and P. eriophaeus are linked by forming groups of collateral capitula in the axils of 15–50 subsequent leaves. (D and E) Paepalanthus subgen. Platycaulon. (D) Sect. Conferti has globose arrangements of sessile capitula on a long cylindrical or slightly flattened peduncle with a basal sheath. (E) In sect. Divisi, all capitula are arranged in one plane; the individual capitula have a distinct short peduncle. The fused part of the peduncle is flat; a sheath at the base may be present or lacking. (F–I) Paepalanthus pruinosus. Development of inflorescences. (F) First the sheath surrounding the entire group of capitula is formed. (G) Slightly later, the primordia of the individual capitula become visible simultaneously. (H) One capitulum seems to be terminal to the entire group. (H and I) The first leaf of each capitulum takes the position of the pherophyll; the second is adorsed to the centre and takes the position which is held by the sheath in E. megapotamicum or S. caulescens. (J–L) Hypothetical transition from a botryoid of capitula to the Platycaulon inflorescence [redrawn after Stützel (1984)]; the prophyll of the entire unit in (E) forms a new sheat; pherophylls and sheaths of the individual capitula become part of the involucrum of the individual capitula; this interpretation is replaced here by a fragmentation of an oversized single capitulum.
Paepalanthus subgen. Platycaulon
Paepalanthus subgen. Platycaulon is characterized by composed capitula in the axils of the leaves of a proliferating rosette. The individual capitula may have a short stalk and are then all in one transverse plane (P. sect. Divisi Koern., Fig. 6E), or the individual capitula are sessile on a joint scape and form a semi-globous to nearly globous arrangement of capitula (P. sect. Conferti Koern., Fig. 6D). For the latter group, the ontogeny has been studied in P. pruinosus Ruhl. (Fig. 6F–I). The formation of the composed capitulum in this group starts from a very large primordium that first forms the transverse truncate sheath (Fig. 6F). The formation of the individual capitula is more or less synchronous (Fig. 6G), and the first leaf to be formed on each of them turns to the periphery of the entire group, and the second to the centre (Fig. 6H, I). Regarding its position, the first leaf corresponds to the pherophyll, and the second one to the prophyll of the individual capitulum. It has to be stressed that the individual capitula lack a pherophyll in most inflorescences of Eriocaulaceae described here, but that it is present in this group. In many species of P. subgen. Platycaulon, there is no vegetative innovation from the rosette. If there is one – as in P. costaricensis Moldenke ex Standl. – it arises from the lower side of the rosette, leading to a cushion-like arrangement of rosettes.
Paepalanthus catharinae Ruhl. and Paepalanthus caldensis Malme
Both species show the same pattern of branching and inflorescence formation. The capitula are solitary in the axils of normal rosette leaves (Fig. 1D, E). During the flowering season, they are formed in a series of consecutive leaves; the closed sheath is always in the adaxial position and represents the prophyll of the pedunculate capitulum. In the axil of those leaves that have not formed capitula after the flowering season, one to several vegetative branches may arise at the lower side of the rosette. This leads to cushion-like groups or flat mats of rosettes.
Paepalanthus distichophyllus Mart. and Paepalanthus stannardi Giul. & L. R. Parra
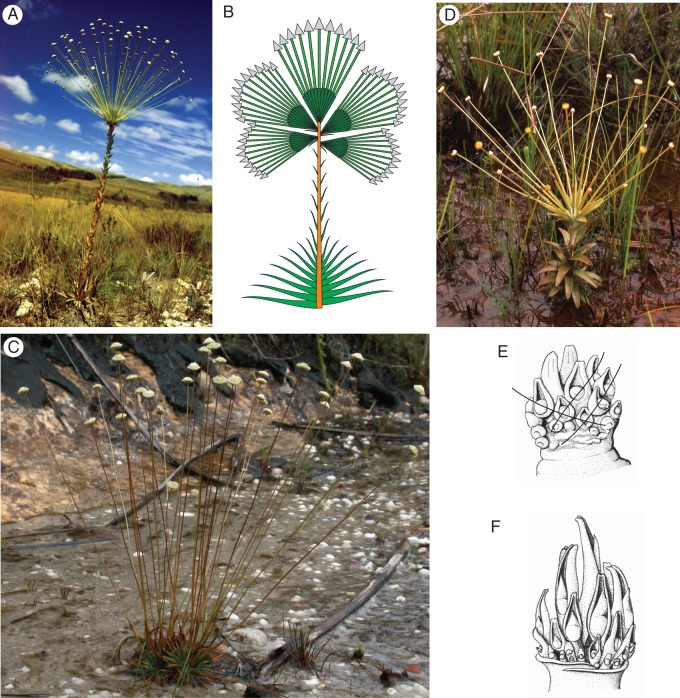
Paepalanthus distichophyllus (Fig. 7D) is a rare species from the Serra do Cipo. It reaches up to 1 m and more, and is extraordinary because of its two types of renewals. The basic arrangement of capitula is a botryoid of very few capitula. Often there are only two capitula or even only a single capitulum. Renewals are formed from the axils of the two leaves directly preceding the stand of capitula. If there are two of these vegetative continuations, it is obvious that the group of capitula or the single capitulum between them is terminal. However, if there is only one vegetative continuation, the capitulum seems to be inserted in an axillary position (laterally, Fig. 7 D, arrow 1). There may be more renewals, often in pairs, only a few internodes below (arrow 2). They are stronger, but if there is only one pair it is the one closest to the stand of capitula. This suggests that these renewals are formed in a basipetal sequence if there are more than two. However, observations are lacking. A further type of renewal originates from the base of the entire plant (arrow 3). It seems that these basal renewals are lateral to a flowering stem growing for several seasons. Paepalanthus stannardii (Fig. 7A–C) shows the same growth mode with at least two different types of innovations. In many respects, this growth type resembles that of small shrubs.
Fig. 7.
(A–C) Paepalanthus stannardii. (A) Lateral renewals below the terminal stand of capitula. (B) M. Trovó in front of a tall branched individual (>2 m). (C) Innovations from the ground, some performing the first terminal inflorescences forcing dichasial or monochasial continuation. (D) Paepalanthus distichophyllus. Arrows indicate (1) a single terminal capitulum having moved into a seemingly lateral position by the vegetative continuation; (2) several paired renewals below a terminal group of capitula; (3) innovations from the ground. (E) Syngonanthus verticillatus is characterized by rosulate leaf clusters separated by scapose internodia on a seemingly monopodial stem. How and why these forms have deviated from the basal rosettes with a terminal botryoid of capitula (as it is typical for the majority of Syngonanthus) is not yet understood.
DISCUSSION
General traits in Eriocaulaceae
Eriocaulaceae show a wide range of different inflorescences (Bongard, 1831; Silveira, 1928). There are also variations within the capitula regarding the distribution of male and female flowers in concentric zones around the apex. The number and size of zones of different sex and thus the relative position of male and female flowers within a capitulum have been reported as relevant for the reproduction mode (Stützel, 1984). The same applies for the duration of anthesis. As flowering and fruiting capitula look the same, notes on herbarium labels such as ‘flowering in May’ generally mean only ‘with capitula’ and do not help to understand the sometimes complex patterns of phenology within capitula or more complex inflorescences. Direct contact of flowering male and female flowers within a capitulum may even lead to autogamy (geitonogamy), despite the strictly unisexual flowers (Stützel, 1981). However, here we focus on the composition of capitula to inflorescences of second and higher order. The known variation in gross morphology has been used to delimit sub-taxa of a different hierarchical level within different genera of Eriocaulaceae (Koernicke, 1863; Ruhland, 1903). Several of these taxa have proven to be monophyletic. On the other hand, some of the remaining taxa are obviously para- or even polyphyletic, and the relationships between accepted groups are badly resolved or totally unclear. At least on the generic and subgeneric level, the detected inflorescence patterns seem to offer meaningful solutions to these difficulties.
According to all information presently available, Xyridaceae (including Abolbodaceae) are the closest relatives to Eriocaulaceae. This is supported by molecular data (Chase et al., 1995; de Andrade et al., 2010) as well as by morphology. Both families have capitula as first-order inflorescences with a strictly acropetal formation of the flowers. Morphological and molecular data are not fully consistent regarding the relationship of Abolboda and Xyris with Eriocaulaceae. It appears settled that Xyridaceae and Eriocaulaceae together form a monophylum. However, as long as some key taxa of both families (Achlyphila, Orectanthe, Mesanthemum and Rondonanthus) are not included in molecular analysis, some important internal nodes cannot be regarded as fully settled. All Xyridaceae and any of the possibly basal Eriocaulaceae have rosettes terminated by a capitulum subtended by a sheath. Additional lateral capitula are formed in a basipetal sequence. For Xyris, this structure has been shown and illustrated by Stützel (1984). The plesiomorphic pattern is thus about the pattern described here for Eriocaulon which also occurs in Mesananthemum, Syngonanthus, Leiothrix and Paepalanthus. Some species in Eriocaulon show all pherophylls of the additional capitula like E. modestum. This is similar to Xyris and thus regarded as primitive here. The lack of pherophylls is a first and early apomorphy within Eriocaulon and the other genera of the family.
While the sequence of formation of the flowers within the capitula is strictly acropetal, the sequence within the simplest stands of capitula is basipetal, and these units always have a terminal capitulum. In Eriocaulon, Syngonanthus and many other species of all other genera in Eriocaulaceae, such units are the only uniformly repeated units. Only in Paepalanthus are these botryoid-like stands of capitula grouped into distinct repetitive units of higher complexity. There are several different architectures for such units of higher complexity. Different groups are characterized by highly diagnostic inflorescence patterns. In P. erectifolius and other species of Paepalanthus sect. Diphyomene Ruhl., the same pattern is repeated again, so that a terminal botryoid of capitula is preceded by several lateral botryoids in a basipetal sequence.
In Actinocephalus, the main axis is not terminated by a flowering unit, but several units of the type of P. erectifolius terminate the first-order lateral axis. These are formed in acropetal sequence. The entire inflorescence thus shows a fifth level of integration. The units of the fourth level are the ball-shaped umbels formed in acropetal sequence which have been termed paracladia by Sano (2004). They are repetitive units in fact, but the term ‘paracladium’ was not used according to Troll (1964). A paracladium sensu Troll should be terminated by a co-florescence repeating the main florescence. However, according to Troll, the florescence would be the capitulum and not a complex arrangement of capitula. In most species of Actinocephalus, the part treated here as a unit of the fourth level blooms in one single flush and there is no innovation from within this unit. Therefore it is treated here as an inflorescence and not as a shoot system with lateral inflorescences. However, minor changes lead to types such as A. robustus, which can be seen perfectly as shoot systems with lateral inflorescences. This underlines that the known variability influences the way to describe the inflorescences. As the homologies between A. polyanthus and A. robustus are obvious and homologous parts should be treated in the same way, they are both described as inflorescences here.
Species with inflorescences similar to A. robustus but with a smaller number of capitula have been collocated in Paepalanthus subsect. Aphorocaulon (Fig. 5B, C) and the first species of the Aphorocaulon group has already been transferred to Actinocephalus (Trovó et al., 2012a) based also on molecular evidence. The much smaller umbels of capitula in this group represent a second-level unit (Fig. 9). It may be derived from a third-level unit by reduction to the terminal second-level unit or by reduction of all second-level units to the terminal capitulum. However, the two options would show the same developmental pattern and thus cannot be distinguished either morphologically or developmentally. This illustrates that typology may lead to useless alternatives.
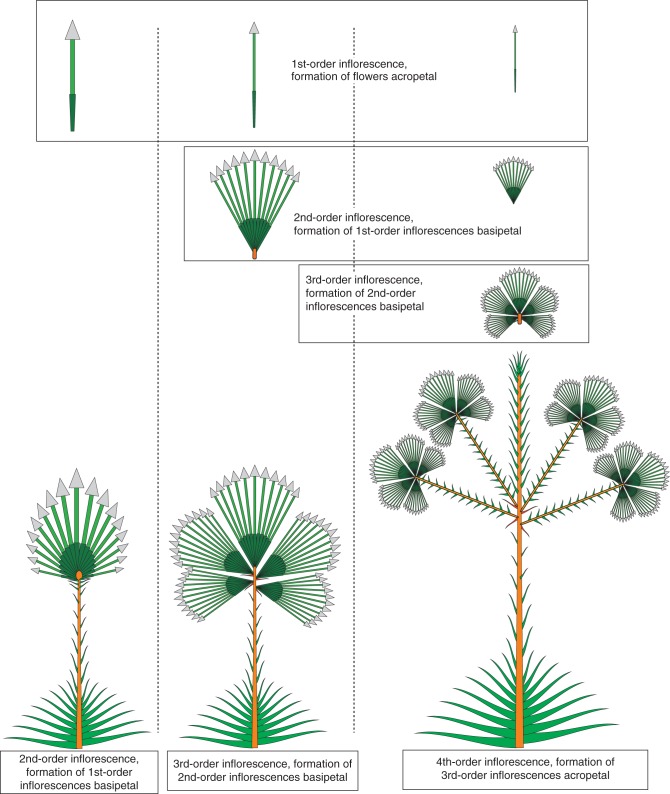
Fig. 9.
In all Eriocaulacae, flowers are arranged in capitulate first-order inflorescences with acropetal formation of the flowers. Capitula are arranged in second-order inflorescences, with basipetal formation of the capitula. In some groups (several) second-order inflorescences are concentrated to distinct third-order inflorescences, the sequence of the second-order units again being basipetal. In the fourth-order inflorescence, the parts are formed in acropetal sequence. As P. catharinae illustrates, the sequence on the second level may also be acropetal. The level of complexity and the sequence of formation are thus independent and useful tools to describe inflorescences of higher complexity.
There seems to be a reduction series from typical representatives of Actinocephalus via species which are presently collocated in Aphorocaulon to species with single axillary capitula such as P. catharinae. Paepalanthus geniculatus with its single capitulum terminating a very short axillary branch is close to bridging that gap. Species such as P. catharinae are presently placed within ser. Variabiles Ruhl. Those species within the section that are characterized by a gynoecium with simple stigmas might be the closest relatives of the Actinocephalus group, which is also characterized by this gynoecium type. However, presently none of these species is included in a molecular analysis. The relationships within Paepalanthus thus remain unclear.
The compound capitula in Paepalanthus subgen. Platycaulon have been regarded as fasciations by Ruhland (1903). This interpretation has been rejected by Stützel (1984) because the known cases of fasciations hardly show the high regularity that is typical for Platycaulon. Stützel has suggested an alternative interpretation (Fig. 6J–L). However, Stützel (1984) could not offer a selective pressure towards such a condensation of capitula. He also ignored the fact that the botryoid stands of capitula in all other groups lack prophylls whereas in this concept they are assumed to be present and transformed into involucral bracts of the entire unit. It is also difficult to imagine how and why a closed sheath should have been lost in one position and formed again de novo at some more basal nodes. Intermediates supporting this interpretation have not been found.
During a field trip to the Chapada de Diamantina in 2009, we discovered a population of Paepalanthus pulchellus Herzog that might help towards a solution of the problem. Paepalanthus pulchellus normally shows a terminal arrangement of capitula (Fig. 8) similar to that in Syngonanthus caulescens. In this population we found several individuals with a hypertrophic terminal capitulum. While the terminal capitulum is otherwise only slightly larger than the basipetally neighbouring ones, the terminal head here was more than three times the diameter of the others. In some individuals, the terminal capitulum was partly sub-divided into distinct capitula that did not form a separate peduncle, but stayed on a joint but rather short peduncle. If the primordium of a capitulum grows so fast that the apex cannot keep control of it, the consequence might be disintegration into morphogenetically independent sub-units. The result would be just what can be seen in Platycaulon.
Fig. 8.
Paepalanthus pulchellus. (A) Normal plant. (B) Individual with a single oversized and sub-sessile capitulum. (C) Oversized terminal unit of several capitula without prominent peduncles (this might be regarded as the result of fragmentation of an oversized meristem leading otherwise to the type in B). (D) Individual with partly sessile and partly pedunculate capitula; as in sect. Divisi, the peripheral capitula are those having the longest peduncles.
Such regular fragmentation of the inflorescence meristem would perfectly agree with the results of the developmental study, but not with the aggregative interpretation (Fig. 6J–L). It is important to note that P. pulchellus is not closely related to Paepalanthus subgen. Platycaulon. The recorded malformations are singular occasional observations. It is not clear if they can be repeated in future fieldwork in so far as they lack essential properties of scientific results. However, these observations can stimulate ideas that could be verified in other species. This would include comparisons of the size of the reproductive apex of the capitulum and stands of capitula. Such comparisons have been shown to be useful for the understanding of simple and compound cones in Taxaceae (Dörken et al., 2011) and might be helpful here, too. Repetitive fragmentations are probably much more common than assumed up to now. The classical morphological approach is to seek the basic units and to understand the complex structure made up of these units in a kind of Lego® construction. While it is mostly easy to find the selective pressure for ‘more and faster’ it is often difficult to imagine a selective pressure for the condensation to super-units of complex structure. The fragmentation approach does not need such a selective pressure as the complex composition is just the result of a developmental repeat in the fragmented sub-units. While the condensation pathway would require at least the temporary existence of intermediates, the fragmentation scenario would allow direct switches without intermediate phenotypes.
Into the fragmentation scenario fit also species such as Paepalanthus polygonus, P. eriophaeus and P. sphaeroides (and species related to the latter; Trovó et al., 2012b). They are somewhat similar to the sect. Divisi of Paepalanthus subgen. Platycaulon, but have the stalks of the individual capitula separate from each other down to the base and each surrounded by a sheath of its own. This can be seen as a fragmentation that occurs earlier in the ontogeny than in subgen. Platycaulon. This concept is morphologically supported by the same floral morphology (bifid stigmas; Ruhland, 1903) and the same type of seed dispersal (catapult mechanism; Trovó and Stützel, 2011). In molecular studies, these taxa are sister to each other as suggested by inflorescence morphology despite being spread over three not closely related groups according to the taxonomy by Ruhland (1903).
Juvenile as well as starving forms of Paepalanthus subgen. Platycaulon show units of only a few or even a single capitulum per peduncle. Such plantlets can thus show the same inflorescence pattern as Paepalanthus catharinae but should have a gynoecium with bifid stigmas. This has been reported for several species of ser. Variabiles (Ruhland, 1903). In contrast to all well-delimited taxa within the family, ser. Variabiles is thus inhomogeneous with respect to this important character. It can be expected that the species of ser. Variabiles have their closest relatives in one of these well-defined subgenera or sections.
In inflorescence morphology ‘reduced’ is generally used in the sense of a morphological comparison. Such a comparison is most easily done by describing the complex inflorescence and mentioning the parts that are lacking in the other, thus more reduced forms. However, this approach does not need to reflect phylogeny, and very often in fact it does not, as the evolution of the complex form cannot be explained in this way. In Eriocaulaceae, it seems to be clear that at least a part of the ‘reduced’ forms such as those in Eriocaulon in fact represent the plesiomorphic condition. The evolutionary pathways led to more complex patterns especially in Syngonanthus and Paepalanthus. By secondary reduction, body plans may result that are similar to the plesiomorphic condition. Species with plesiomorphic simple and secondary simple inflorescence types are most probably classified together in Paepalanthus ser. Variabiles. Molecular studies reflect this idea only partly as species showing the simple patterns are under-represented in these studies (de Andrade et al., 2010; Parra et al., 2010; Trovo et al., 2013).
In proliferating capitula, the prolification is always terminal due to a stepwise reversal from the formation of floral bracts to enlarged and green leaves. This reversal is accompanied by a stepwise reduction in the size of the apex and by a change of its form from slightly cup-like to cone-shaped. This type of prolification has been described for many species, but probably only for some species of Leiothrix is this a constant and diagnostic feature. It has to be stressed that prolifications may occur independently on any level of complexity. These prolifications may be, but do not need to be, a ‘reversal to vegetative growth’ (Weberling, 1981). They may also simply describe an ongoing vegetative growth (Figs 1D, 4E, 5A and 6A, B). This may obscure the distinction of the vegetative part and the inflorescences. Especially if broader comparisons are undertaken, parts have to be included which would be regarded otherwise as part of the vegetative zone. This applies, for example, for Eriocaulon megapotamicum.
The comparison within Paepalanthus also illustrates that a definition of ‘innovation’ or ‘renewal’ is problematic. Paepalanthus distichophyllus shows a kind of innovation from the tip of aerial flowering shoots. The branching pattern of these parts is similar to the pattern of the rosette in many species of Eriocaulon. The renewals from the base are different and more similar to the shoots coming from the base of a shrub. However, it is unknown if the basal renewals appear each year or – similar to some shrubs – only at irregular intervals. Such different innovations have been addressed probably for the first time by Claßen-Bockhoff (2000) in the woody Bruniaceae. Comparisons can be restricted to the variable elements without losing information. The reference system (see Claßen-Bockhoff, 2000) may therefore vary according to size and variability of the taxon under study. It can be smaller within closely related groups and often needs to be larger in major or variable groups. Too small reference systems (or too small herbarium specimens) may result in completely misleading descriptions. This is best illustrated by the excellent work by Silveira (1928) based on his own field studies. However, most of his new species later turned out to be younger synonyms of species already described incompletely and misleadingly by others based on parts of the plants which were too small. The appropriate reference system obviously cannot be detected by applying a fixed or standardized procedure, but is the result of research of its own.
The mutual support of floral morphology, inflorescence morphology and molecular data is especially important if one type of data is not accessible. Due to microendemism with a sometimes very narrow range, several species of Eriocaulaceae are known only from the type specimen, which may be too old or too small and precious to extract the relevant markers. In the field, floral characters are hardly useful due to the minute size of the flowers, but inflorescence characters are (relatively) easily accessible field characters. On the other hand, in really tall herbarium specimens, only restricted parts of the entire plant are represented. Molecular data together with data on floral morphology will allow a very good idea about habit and inflorescence morphology to be obtained even in incompletely known species. This is important if such species have to be re-collected. In the latest monograph on the entire Eriocaulaceae (Ruhland, 1903), about one-third of the genus Paepalanthus forms the heterogeneous conglomerate of series Variabiles. Inflorescence morphology together with other morphological characters will be important to decide which species will have to be re-collected for molecular analysis and for which species morphological data might be sufficient. In this context it has to be stressed that neither sequencing nor handling large data sets is a limiting factor any longer. However, it is important to focus the limited resources for field work and collecting activities on those taxa which seem to be most relevant to detect phylogenetic relationships. Furthermore, it must be stressed that the quality of molecular trees depends largely on proper identification of the specimens used and that morphology remains crucial in this respect.
The Troll approach and the approach used here
In addition to floral morphology, the morphology of inflorescences has always been used in plant taxonomy. As there is much more intraspecific variability in the structure of inflorescences than in that of flowers, inflorescence morphology always appeared more complex and less uniform than floral morphology. Earlier attempts are summarized in Eichler (1875) and in Weberling (1989). The most comprehensive treatment of inflorescences is still that by Troll (1964, 1969), Weberling (1981, 1989), Troll and Weberling (1989) and Weberling and Troll (1998). The number of terms introduced by Troll to describe inflorescences grew continuously without making the approach more powerful in plant taxonomy. This is partly because the Troll morphology was intended to be diagnostic in establishing distinct types rather than being used for describing continuous evolutionary processes (Kunze, 1989). Later additions in theory and terminology appear to be partly a kind of ‘repair’ of weaknesses of the earlier concept.
Troll clearly has outstanding merits in making inflorescence morphology more precise and more useful in taxonomy. It is obvious that he started his work analysing annual plants. By doing so, he could base his comparisons on entire plants and did not have to select parts. This is the easiest way to avoid comparisons being based on non-homologous parts. When proceeding to perennial plants, he decided to use the annual growth unit (‘Jahrestrieb’) as the basis for comparisons. In the meantime, it has been demonstrated that this may lead to problems even in Europe when comparing taxa with species that moved from a Mediterranean climate with a winter vegetation period and draught rest in summer to the northern temperate zone with summer vegetation and cold rest in winter (Werner and Ebel, 1994). Briggs and Johnson (1979) have therefore replaced the annual growth unit by the seasonal growth unit (SGU). This works nicely in regions with clearly distinct seasons and for taxa that did not shift between regions with different climatic regimes or seasons during their evolution. The modified Briggs and Johnson approach is thus also only of limited use in regions with highly variable climatic conditions where two or more growing seasons may be incompletely separated by dry or/and cold phases. Our approach demonstrates that inflorescence morphology can be used as a powerful tool even if it is not possible to delimit clear SGUs.
Endress (2010) has clarified much of the previous terminological confusion. He already stated that the Troll terminology and some of the later refinements appear oversophisticated and sometimes hinder a clear understanding rather than help towards it. Our approach is therefore intended to be as clear as possible with as few terms as possible. We search for distinct and repetitive units on different levels of complexity. For each level we describe the kind of units formed (flowers, inflorescences of first, second, third …. order), the branching pattern within the unit and the sequence of formation of the respective units (Fig. 9). In Fig. 9 only hapaxanth species are represented, and the position and time lag between subsequent growth units are therefore lacking. However, it is possible to include that also in graphic representations. The given examples show that the level of complexity or aggregation and the formation sequence are independent features. The examples presented here demonstrate that the (repeated) combination of both features leads to a relatively simple description of highly complex inflorescences.
The patterns described in this way have been shown also to be relevant to detect evolutionary patterns. The delimitation of the synflorescence seems to be problematic even for comparisons within Eriocaulaceae. Therefore, we do not see a significant advantage of the synflorescence concept compared with the traditional inflorescence and abandon the term. Instead we try to identify larger time lags in the development of the flowering parts. For species for which only a few and isolated records are available (e.g. P. distichophyllus), data in this respect remain scarce. The parts formed between such vegetative lag phases may be distinct SGUs as defined by Briggs and Johnson (1979), but for many Eriocaulaceae this is not yet clear at all.
Troll (1964, 1969) stressed that two types – monotelic and polytelic – should be kinds of primary types, not linked at all; however as pointed out by Endress (2010), this concept is in conflict with an evolutionary approach to inflorescences. Sell (1969) has described an evolutionary pathway leading from monotelic to polytelic inflorescences by racemization and truncation.
He had already recognized that similar processes may be repeated on higher levels of complexity. His ‘double truncation’ is a kind of first step towards the secondary polytely as proposed by Kunze (1989). Sensu Troll, Eriocaulaceae are strictly polytelic as the first-order inflorescences (capitula) are always racemes (or racemous capitula) and thus indeterminate. However, on the seceond level of complexity we can find determinate units (e.g. S. caulescens) as well as indeterminate ones (e.g. P. catharinae). For these second-level inflorescences Troll used the term pseudoflorescences, with the paracladia terminating in pseudoflorescences he called long-paracladia (in contrast to the normal short-paracladia). The botryoid of capitula as described for E. megapotamicum and S. caulescens would represent such long-paracladia or pseudoflorescences. This strategy works for a second level of complexity. As shown, for Eriocaulaceae five levels of complexity would be needed. The same applies for grasses and many other taxa and, following the Troll strategy, further terms would be needed for units of higher complexity. However, it seems to be easier and clearer to number the levels of complexity consecutively and to describe the ramification pattern and formation sequence separately for each of them.
If there is a terminal unit for a given level of complexity, it is flowering earlier than the preceding ones. However, if there is no such terminal unit, initiation as well as the flowering sequence of these units is strictly acropetal. The pattern described as monotelic and polytelic by Troll (1964, 1969) for flowers is thus repeated on higher levels of complexity (capitula, racemes, thyrses or cyathia) in the same way. Schröder (1987) as well as Kunze (1989) have already pointed out the importance of such repetitive units on different complexity levels, and Schröder (1987) has even termed them ‘modules’. Rua (1996) and Pensiero and Vegetti (2011) faced similar difficulties when treating grass inflorescences. They dealt with grasses as if the spikelets were flowers. Otherwise they treated Setaria and Paspalum in the tradition of the Troll school without defining a hierarchical set of ‘modules’ or ‘units’. The similarities of patterns for second-order inflorescences described here for S. caulescens, A. erectifolius and others to the pattern described for Bruniaceae, especially Linconia alopecuroides (Claßen-Bockhoff, 200; p. 104), is striking despite the difference in the first-order inflorescences. This also underlines that it makes sense to analyse the complexity levels separately.
The double truncation as described by Sell (1969) and Kunze (1989) seems to be understood by both as two subsequent steps of loss. The fragmentation process as described for Paepalanthus subgen. Platycaulon suggests that there was never a complex terminal unit being lost in several steps. Instead, there was a step towards a higher complexity of the lateral branches. For a merely typological comparison in the Troll tradition, this would not make a difference. For an understanding of the evolutionary radiation, it is, however, essential.
Troll did not analyse meristem sizes, and the treatment of Bruniaceae by Claßen-Bockhoff (2000) is predominantly based on herbarium material and field work, and therefore also lacks studies of meristem sizes. Kunze (1989) used SEM studies only to detect the relative position of parts within an inflorescence. In the present study, the SEM technique was used also in the classical context, but the results showed that there are significant differences in size correlations. A comparative study of meristem properties for vegetative and inflorescence meristems should be undertaken for units of different complexity to achieve a better understanding of inflorescence evolution.
There is another interesting logical break in the terminology of the Troll school (and precursors). They define compound inflorescences ‘if the individual flowers of simple inflorescences (raceme, spike, umbel, and capitulum) are replaced by a complete inflorescence of the same branching type, then certain types of compound inflorescences, the double raceme [diplobotryum …], double spike, double umbel (…), and the double capitulum are obtained’ (Weberling, 1989; p. 207) (the German text from 1981 uses the term ‘dibotryum’ instead of ‘diplobotryum’). However, the dithyrse is not a thyrse in which the flowers are replaced by entire thyrses, but a raceme or botryoid in which all flowers are replaced by a thyrse (Weberling, 1989; p. 216). The description of the simple branching pattern is obscured by the introduction of further and unnecessary terms such as ‘Spezialthyrsus’ (erroneously translated as ‘sub-thyrse’ in Weberling, 1989). Weberling shows in his illustration of a pleiothyrse that the units appearing as compact and thus distinct sub-units may represent different levels of complexity. In his illustration (Weberling, 1989; p. 217) the sub-units of the entire inflorescence being distinct in habit or appearance are already dithyrses. In our way of describing inflorescences, this would correspond to an inflorescence of the fourth order, the first-order unit representing a cyme, and the second- to fourth-order units all showing the pattern of a raceme.
Troll already realized that the condensation to visually distinct units may occur on different levels of complexity. For this aspect, Troll used the terms ‘heterocladic’ and ‘disjunct heterocladic’. However, it is possible to describe these facts without introducing any new term. The occurrence of habitually distinct units allows a morphologically precise (typological) description for the arrangement of those units to be mixed a with a merely superficial description of the pattern within these units. Especially in floristics, key-making and related fields this can be a meaningful approach, whereas evolutionary studies as well as developmental genetics would require a precise (typological) approach throughout. If typological and descriptive approaches are mixed, it is important to indicate clearly which parts are understood throughout and for which parts only gross morphology is described. A comparison, e.g. within Actinocephalus, does not require a full understanding of the branching pattern within the ball-shaped umbels of capitula, but a comparison within the entire family does.
Conclusions
Analysing inflorescences in the Troll/Weberling manner is a powerful tool in taxonomy. It can be done with much less terminological burden than proposed by Troll without losing its strength. This is especially the case if there are distinct levels of complexity in the inflorescence pattern within a group. In this case, the following parameters can be described separately:
the pattern of ramification for each complexity level (branches bearing flowers, inflorescences of first, second, third, … order);
the sequence of formation of the units of each complexity level;
the description of those parts (levels) of the inflorescence which form distinct units in habit and function;
the time lag between subsequent complexity levels to allow estimation of the possible delimitation of a seasonal growth unit.
ACKNOWLEDGEMENTS
The present work is based mainly on fieldwork which would not have been possible without the support of many people. First studies were carried out during a joint field trip of T.S. to Serra do Cipo guided by Ana Maria Giulietti as early as 1987. Additional data were collected on field trips by M.T. together with Livia Echternacht (who also supplied photos and morphological information from her own field trips), T.S. together with Alessandra Ike-Coan and Aline Oriani, and all of them together. We are grateful for financial support to T.S. from the DFG and the Humboldt foundation, and to M.T. from FAPERJ, CAPES and the Humboldt foundation. Without the slight but continuous pressure by R. Claßen-Bockhoff on T.S., the manuscript would probably never have been finished. Major reorganization of a previous version of the manuscript would not have been possible without the help of N. Balnis and S. Adler. Last but not least we are grateful to Simon Mayo for liguistic adjustments and for many comments and fruitful discussions.
LITERATURE CITED
- de Andrade MJG, Giulietti AM, Rapini A, et al. A comprehensive phylogenetic analysis of Eriocaulaceae: evidence from nuclear (ITS) and plastid (psbA-trnH and trnL-F) DNA sequences. Taxon. 2010;59:379–388. [Google Scholar]
- Bongard M. Essai monographique sur les espèces d' Ériocaulon du Brésil. Mémoires de l'Academie Imperiale des Sciences de Saint-Petersbourg. Sixieme Serie. Sciences Mathematiques, Physiques et Naturelles. 1831;1:601–655. [Google Scholar]
- Briggs BG, Johnson LAS. Evolution in the Myrtaceae – evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales. 1979;102:159–256. [Google Scholar]
- Chase MW, Stevenson DW, Wilkin P, Rudall PJ. Monocot systematics: a combined analysis. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. Kew: Royal Botanic Gardens; 1995. pp. 685–730. [Google Scholar]
- Claßen-Bockhoff R. Inflorescences in Bruniaceae. Opera Botanica Belgica. 2000;12:5–310. [Google Scholar]
- Dörken V, Zhang Z, Mundry I, Stützel T. Morphology and anatomy of male cones of Pseudotaxus chienii (W.C. Cheng) W.C. Cheng (Taxaceae) Flora. 2011;206:444–450. [Google Scholar]
- Eichler AW. Blüthendiagramme. Leipzig: Engelmann; 1875. [Google Scholar]
- Endress PK. Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics and Evolution. 2010;48:225–239. [Google Scholar]
- Gerstberger P, Leins P. Rasterelektronenmikroskopische Untersuchungen an Blütenknospen von Physalis philadelphica (Solanaceae) – Anwendung einer neuen Präparationsmethode. Berichte der Deutschen Botanischen Gesellschaft. 1978;91:381–387. [Google Scholar]
- Giulietti AM, Hensold NC. Synonymization of the genera Comanthera and Carptotepala with Syngonanthus (Eriocaulaceae) Annals of the Missouri Botanical Garden. 1991;78:460–464. [Google Scholar]
- Koernicke F. Eriocaulaceae. In: von Martius CP, Eichler AW, editors. Flora Brasiliensis. 1. Vol. 3. 1863. pp. 273–307. München: Typographia Regia. [Google Scholar]
- Kunze H. Probleme der Infloreszenztypologie von W. Troll. Plant Systematics and Evolution. 1989;163:187–199. [Google Scholar]
- Parra LR, Giulietti AM, de Andrade MJG, van den Berg C. Reestablishment and a new circumscription of Comanthera (Eriocaulaceae) Taxon. 2010;59:1135–1146. [Google Scholar]
- Pensiero JF, Vegetti AC. Inflorescence typology in Setaria P. Beauv. (Poaceae, Paniceae) Feddes Repertorium. 2011;112:371–385. [Google Scholar]
- Prenner G, Vergara-Silva F, Rudall PJ. The key role of morphology in modelling inflorescence architecture. Trends in Plant Science. 2009;14:302–309. doi: 10.1016/j.tplants.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Rua GH. The inflorescences of Paspalum (Poaceae, Paniceae): the Quadrifaria group and the evolutionary pathway towards the fully homogenized, truncated common type. Plant Systematics and Evolution. 1996;201:199–209. [Google Scholar]
- Ruhland W. Eriocaulaceae. In: Engler A, editor. Das Pflanzenreich, Regni vegetabilis conspectus IV. 30 (Heft 13: 1–94). W. Engelmann: Leipzig; 1903. [Google Scholar]
- Sano PT. Actinocephalus (Körn.) Sano (Paepalanthus sect. Actinocephalus), a new genus of Eriocaulaceae, and other taxonomic and nomenclatural changes involving Paepalanthus Mart. Taxon. 2004;53:99–107. [Google Scholar]
- Schröder F-G. Infloreszenzen, Synfloreszenzen und Moduln. Ein terminologischer Beitrag zur Infloreszenzmorphologie. Botanischr Jahrbücher für Systematik. 1987;108:449–471. [Google Scholar]
- Sell Y. Les complexes inflorescentiels de quelques Acanthacées. Étude particulière des phénomènes de condensation de racemisation et de troncature. Annales des Sciences naturelles – Botanique et biologie ve'ge'tale. 12e série. 1969;10:225–300. [Google Scholar]
- Silveira AA. Floralia Montium. Vol. 1. Belo Horizonte: Impresa Official; 1928. [Google Scholar]
- Stützel T. Zur Funktion und Evolution köpfchenförmiger Blütenstände, insbesondere der Eriocaulaceen. Beiträge zur Biologie der Pflanzen. 1981;56:439–468. [Google Scholar]
- Stützel T. Blüten- und infloreszenzmorphologische Untersuchungen zur Systematik der Eriocaulaceen. Dissertationes Botanicae. 1984;71 Vaduz: Verlag J. Cramer. [Google Scholar]
- Troll W. Die Infloreszenzen Bd. I. Stuttgart: Gustav Fischer Verlag; 1964. [Google Scholar]
- Troll W. Die Infloreszenzen Bd. II-1. Stuttgart: Gustav Fischer Verlag; 1969. [Google Scholar]
- Troll W, Weberling F. Infloreszenzuntersuchungen an monotelen Familien. Stuttgart: Gustav Fischer Verlag; 1989. [Google Scholar]
- Trovó M, Stützel T. Diaspores in Eriocaulaceae: morphology, mechanisms, and implications. Feddes Repertorium. 2011;122:456–464. [Google Scholar]
- Trovó M, Stützel T. On the morphological position of Paepalanthus subgenus Psilandra (Eriocaulaceae) Plant Systematics and Evolution. 2013;299:115–121. [Google Scholar]
- Trovó M, Stützel T, Scatena VL, Sano PT. Morphology and anatomy of inflorescence and inflorescence axis in Paepalanthus sect. Diphyomene Ruhl. (Eriocaulaceae, Poales) and its taxonomic implications. Flora. 2010;205:242–250. [Google Scholar]
- Trovó M, Costa FN, Echternacht L. Actinocephalus pachyphyllus: re-establishment, redefinition, and a new combination in Eriocaulaceae from Brazil. Kew Bulletin. 2012a;67:25–31. [Google Scholar]
- Trovó M, Echternacht L, Sano PT. Paepalanthus sphaeroides, a new species of Eriocaulaceae. Blumea. 2012b;57:105–108. [Google Scholar]
- Trovó M, de Andrade MJG, Sano PT, Ribeiro PL, van den Berg C. Molecular phylogenetics and biogeography of Neotropical Paepalanthoideae with emphasis on Brazilian Paepalanthus (Eriocaulaceae) Botanical Journal of the Linnean Society. 2013;171:225–243. [Google Scholar]
- Van Stenis CGGJ. Definition of the concept ‘inflorescence’ with special reference to ligneous plants. Flora Malesiana Bulletin. 1963;18:105–1007. [Google Scholar]
- Weberling F. Morphologie der Blüten und der Blütenstände. Stuttgart: Verlag Eugen Ulmer; 1981. [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Weberling F, Troll W. Die Infloreszenzen Bd. II-1. Typologie und Stellung im Aufbau des Vegetationskörpers. Jena: Gustav Fischer Verlag; 1998. [Google Scholar]
- Werner K, Ebel F. Lebensgeschichte der Gattung Helleborus (Ranunculaceae) Flora. 1994;189:97–130. [Google Scholar]