Fig 4.
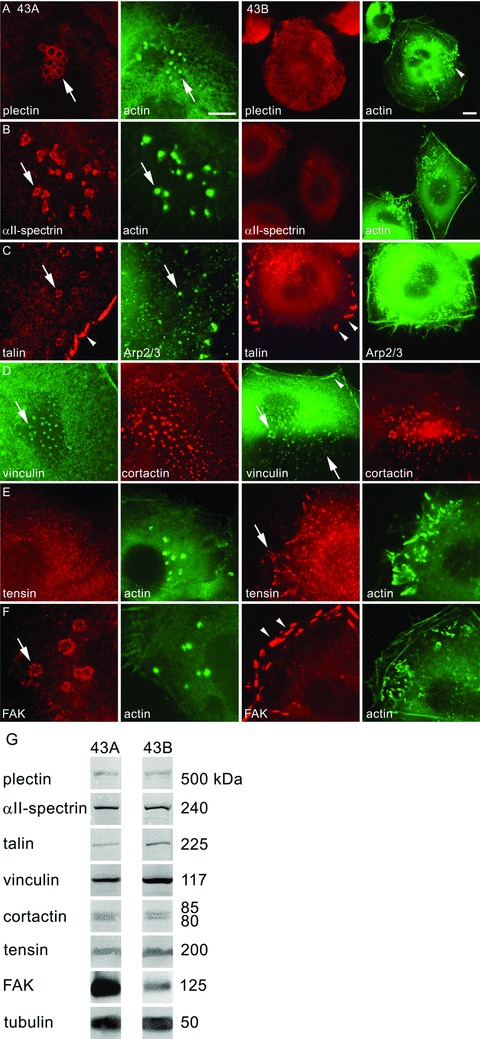
Localization of plectin, αII-spectrin, talin, vinculin, tensin and FAK, in 43A podosome-like structures and 43B invadopodia. Plectin immunoreactivity localized in peripheral rings of podosomes in EGFP-actin transfected 43A cells (A, arrow). However, in 43B cells, plectin did not localize to invadopodia that were characterized by EGFP-actin accumulation (arrowhead), but associated with cytoplasmic filaments. αII-spectrin immunoreactivity was found in the peripheral rings surrounding the podosome cores (B, arrow). In 43B cells, αII-spectrin was found as diffuse cell surface immunoreactivity. In double-labelling with Arp 2/3 (C), talin was detected both in podosome rings (arrow) and focal adhesions (arrowhead) in 43A cells, whereas in 43B cells, talin was found only in focal adhesions (arrowheads). Vinculin colocalized in double-labelling with cortactin in podosome cores, invadopodia and club-ended cell extensions (D, arrows). Vinculin immunoreactivity was found also in focal adhesions in 43B cells (arrowhead). In 43A cells, tensin reactivity was diffuse, but in 43B cells, it localized to invadopodia (E, arrow). FAK was confined to the peripheral ring of podosomes (F, arrow), but in 43B cells it localized only to focal adhesions (arrowheads). Scale bars: 10 μm. In Western blots of 43A and 43B cell lysates, the protein levels of plectin, αII-spectrin, talin, vinculin, cortactin and tensin were equal (G). In immunoprecipitation with Mab against FAK followed by Western blot with MAb against phosphotyrosine, FAK expression levels were somewhat stronger in 43A cells compared with 43B cells. Tubulin was used as a loading control.
