Fig 5.
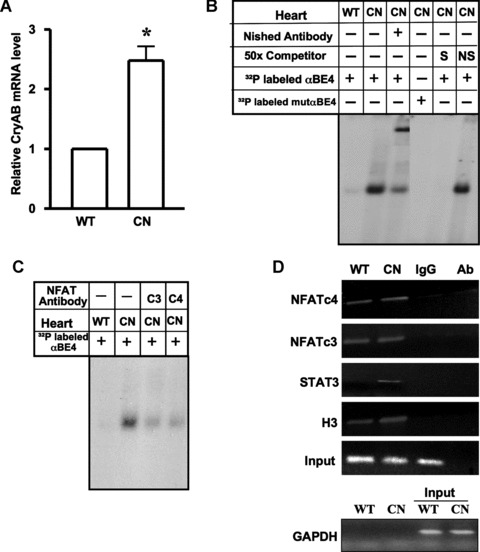
MHC-CnA mice have enhanced levels of CryAB mRNA and induced αBE-4/protein complex formation. (A) Real time PCR was done for quantification of CryAB mRNA in the hearts of WT C57BL/6 (WT) (n= 7) and transgenic MHC-CnA (CN) (n= 7) mice. β-actin was used for normalization. Data are mean ± SE. *P < 0.05. (B) Nuclear extracts were prepared from hearts of WT and MHC-CnA (CN) littermates. Gel mobility assays were performed with radiolabeled αBE4 or mutated αBE4 oligonucleotides. Binding reactions were performed in the presence of anti-Nished antibody, 50x molar excess unlabeled αBE4 (S) and nonspecific (NS) oligonucleotides. (C) Preincubation of cardiac nuclear extracts isolated from MHC-CnA mice (CN) with anti-NFATc3 (c3) and c4 (c4) antibodies were shown. Data shown are representative of three separate experiments, with two individual mice per experiment. (D) Chromatin isolated from WT and MHC-CnA mice (CN) hearts was subjected to ChIP. The chromatin DNA was immunoprecipitated with antibody against NFATc4, c3, STAT3 and acetylated histone H3 and analyzed by semiquantitative PCR with primers flanking the αBE4 sequence. A non-specific IgG was used as a negative control for immunoprecipitation and antibody alone (Ab), without lysate, served as a control for cross-contamination. Input DNA represents 10% of total chromatin used in each reaction. Primers specific to GAPDH were used before (input) and after immunoprecipitation as a control to monitor immunoprecipitation specificity. Data shown are representative of three separate experiments, with two individual mice per experiment.
