Abstract
The collagenolytic effects of the tissue-type plasminogen activator (t-PA) leading to extracellular matrix degradation are clearly involved in the physiopathology of human foetal membranes rupture. Nevertheless, the regulation of t-PA gene expression in extraembryonic developmental contexts remains unknown. The aim of our study is to propose the retinoic acids (RAs) as molecular regulators of t-PA expression in foetal membranes. RA induced t-PA mRNA and proteins in a time-dependent manner in amniotic membrane explants and Wistar Institute Susan Hayflick (WISH) cells. Furthermore, the use of cycloheximide revealed a two-step regulation of t-PA gene. Gene reporter assays confirmed that the RA-induced t-PA gene expression occurred through interactions of retinoid receptors (RARs and RXRs) with a DR5 response element located at –7 kb from the transcription site. Site-directed mutagenesis of this region of the t-PA promoter showed that SP1 factor was also retinoid-mediated induction, and immunoprecipitation assays revealed that SP1 and RAR/RXR interacted physically. Chromatin immunoprecipitation demonstrated that interactions between RARs, RXRs and t-PA promoter were time dependent: RAR-α/RXR-α bound DR5 motif before and up to 12 hrs of RA exposure, and RAR-β/RXR-α bound DR5 response element after 12 hrs of RA treatment. Finally, experiments using shRNA and RAR-β-specific antagonist revealed that reducing RAR-β induction decreased t-PA induction. Altogether, our results established that the RA-mediated regulation of t-PA in human foetal membranes occurred through two steps, with a major role played by RAR-β.
Keywords: human, foetal membranes, amnion, retinoids, tissue-type plasminogen activator, retinoic acid receptor beta
Introduction
Gene regulation needs to be harmoniously controlled to allow precise appropriate mammalian development of embryonic and extraembryonic structures such as the placenta and foetal membranes. The amnion and chorion are foetal membranes involved in many physiological processes during pregnancy, such as protection and nutrition of the developing foetus, amniotic fluid homeostasis and parturition. These membranes form a highly specialized interface between mother and foetus and are absolutely essential to an optimal pregnancy outcome. They could also be involved in human obstetrical pathologies such as chorioamnionitis, oligohydramnios or pre-term pre-labour rupture of membranes (PPROM) [1]. The amnion consists mostly of a single layer of epithelial cells (amniocytes) on a thick basement membrane and a spongy collagen layer containing mesenchymal cells [2]. The chorion is a more opaque membrane attached to the maternal decidua containing trophoblastic cells. These layers mainly comprise extracellular matrix (ECM) proteins such as different collagen types, laminin, fibronectin and proteoglycans [3]. This complex framework allows optimal membrane resistance during pregnancy by increasing both the tensile strength and the elasticity of the foetal membranes [4]. During or just prior to labour, the breakdown of these proteins is regulated by the matrix metalloproteinases (MMPs) system [5]. These biochemical changes and the architectural disorganization of the foetal membranes reduce their integrity and elasticity, leading to membrane weakening and rupture, heralding the initiation of parturition [6].
The plasminogen activator system, which includes tissue-type plasminogen activator (t-PA), plays an important role in the regulation of MMP activities [7, 8], leading to plasminogen activation and conversion into plasmin. This gives rise to the activation cascade of latent proenzymes such as pro-MMPs in a phenomenon that leads to ECM degradation. By controlling the turnover of collagens, t-PA is one of the most important actors in the ECM degradation of human foetal membranes. Indeed, t-PA gene expression increases in foetal membranes after pre-term labour and delivery [9, 10]. The molecular mechanism regulating t-PA expression in amniotic membranes remains unknown. However, retinoic acid (RA) increases t-PA expression in human umbilical vein epithelial cells (HUVEC) [11], blood mononuclear cells [12], astrocytes [13], teratocarcinoma [14], fibrosarcoma and osteocarcinoma cell lines [15].
Retinoids, including retinol (vitamin A) and its active metabolites all-trans and 9-cis retinoic acid (atRA and 9cisRA, respectively), play an important role in the control of cell proliferation and differentiation, particularly during embryonic and placental development [16]. Retinoids mediate their action by binding two families of nuclear receptors named retinoic acid receptors, or RARs (RAR-α, -β and -γ), and retinoid X receptors, or RXRs (RXR-α, -β and -γ). These receptors act as ligand-activated transcription factors and form heterodimers RAR/RXR binding DNA response element (RARE) located on specific target genes [17]. The implications of retinoids in terms of placental development and physiology have been clearly established [18]. Moving on from our previous demonstration that the molecular and metabolic actors of retinoid signalling pathways are functional in human foetal membranes [19], the first aim of our study was to establish the regulation of t-PA gene expression by RA in this extraembryonic environment. The second aim of this study was to identify the different actors involved in this amniotic retinoid regulation of t-PA.
Materials and methods
Chemicals and reagents
atRA, 9cisRA, cycloheximide (CHX), trypsin, protease inhibitors and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich® (Lyon, France). LE135 RAR-β-selective antagonist [20] was obtained from Tocris® (Bristol, United Kingdom). The culture medium and additives (streptomycin and penicillin) were acquired from Invitrogen® (Cergy-pontoise, France), and dextran-coated charcoal-stripped foetal calf serum (FCS) was purchased from ATGC® (Marne la Vallee, France). The transfection reagent GeneJammer was obtained from Agilent Technologies®, Massy, France. BAC of chromosome 8 (RP11–231D20) containing t-PA gene was acquired from Roswell Park Cancer Institute® (Buffalo, NY, USA).
Tissue collections
Human foetal membranes were obtained from 15 different patients with healthy pregnancy (38.0 ± 0.5 weeks of gestation) undergoing planned caesarean section (Hôtel-Dieu Maternity, Clermont-Ferrand, France) after gaining informed consent in accordance with the Declaration of Helsinki and institutional ethic committee. Placental tissues and amniotic membranes were immediately used for stimulation by retinoids and/or were frozen at −80°C for RT-PCR and protein assays. To obtain reproducible results, the amnion explants were always taken from the same location, as recommended previously [21].
Cell and tissue culture
Both the amnion explants and the human amnion-derived Wistar Institute Susan Hayflick (WISH) epithelial cell line cultures were conducted as previously described [19].
Quantitative RT-PCR experiments
Total RNA was extracted from human total amnion, chorion and cell cultures using TRIZOL (Invitrogen®). The cDNA synthesized from 2 μg of RNA was generated using a Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen®). RT-PCR reactions were performed with the DNA Master SYBRGreen I® reagent set in the Light Cycler® system (Roche Diagnostics®(Meylan, France)). Quantification of the housekeeping gene acidic ribosomal phosphoprotein P0 (36B4) transcripts was performed for all samples as an internal control on the amount and quality of cDNA [19]. The results were given as the ratio between t-PA and 36B4 transcripts. All experiments were performed in triplicate. PCR products were checked on a 1.5% agarose gel. The primer sequences used for the analysis are described in Table 1.
Table 1.
Sequence of primers used for expression analysis (upper part) and for mutagenesis (lower part)
| Forward | Reverse | Size (pb) | |
|---|---|---|---|
| Primers for expression analysis | |||
| t-PA | 5′ -CTGGGGAACC AC AACT AC-3′ | 5′-GTTCTGTGCTGTGTAAACCT-3′ | 243 |
| SP1 | 5′-TGTAAAGACAGTGAAGGAAG-3′ | 5′-GTGGGTCTTGATATGTTTTG-3′ | 200 |
| 36B4 | 5′ -GACCTGGAAGTCC AACT ACT-3′ | 5′-GTGATATCAAGCACTTCAGG-3′ | 600 |
| XDR5t-PA | 5′ -AGGTCTGAGTGATCTCATTG-3′ | 5′-ACAAT AACC AAAACCAAGTG-3′ | 151 |
| XDR5RAR-β | 5′ -CTCTCTGGCTGTCTGCTTTT-3′ | 5′-GGCAAAGAATAGACCCTCCT-3′ | 233 |
| Primers for mutagenesis | |||
| t-PA-0,4 DR5mut | 5′– GCC ATGGCCTGGGACTCTGGGTA TTACGCACTAAACGAAGGAATT ATC-3′ | 5′-CAGAGTCCCAGGCCATGGCTGT GTCTGGGGCG-3′ | |
| t-PA-0,4 DR5SPlmut | 5′-CTTTGGCCGCTCTCCCAAAGGGATCGTTACTAGACACAGCCATGG-3′ | 5′-CCTTTGGGAGAGCGGCCAAAGCCCTATTCACCTCG-3′ | |
t-PA immunohistological and -cytological staining assays
Cryosections of the whole amnion, chorion and WISH cells grown in Lab-Tek culture chambers (MC2®(Clermont-Ferrand, France)) were fixed in 4% paraformaldehyde in PBS (pH 7.4) at room temperature (RT) for 10 min., rinsed three times with PBS, incubated at RT for 10 min. in H2O2 (quenching of endogenous peroxidases) and incubated in PBS with 3% bovine serum albumin at RT for 30 min. The cells and tissues were incubated overnight at 4°C in the presence of t-PA certified goat polyclonal primary antibody (American Diagnostica®(Neuville-sur-Oise, France), 1/200 in PBS). This step was followed by three PBS washes and a 1-hr incubation in the presence of a secondary donkey polyclonal HRP anti-goat antibody (Abcam® (Paris, France)) at RT (dilution in PBS 1/5000), followed by three washes with PBS. The samples were then mounted in an aqueous propyl gallate/PBS mounting fluid and examined by DAPI nuclear staining (5 min., dilution in PBS 1/5000) under a Zeiss® (Le Pecq, France) Axiophot microscope. For negative controls, the sections were incubated without primary antibody.
t-PA protein quantification
The levels of t-PA (ng/ml) expressed in control and retinoid- stimulated foetal membranes and WISH cells were determined using the Imubind t-PA ELISA kit (American Diagnostica®). ELISA experiments were performed according to the manufacturer’s instructions. For each condition, t-PA protein concentration was normalized by total cellular protein concentration measured by the Biuret method on a Modular P800 analyser (Roche Diagnostics®).
Plasmid construction
For t-PA promoter analysis (see Fig. 2A), the different constructs (pt-PA1.0-CAT, pt-PA4.8a-CAT, ptPA4.8b-CAT, pt4.8c-CAT and pt1.4-CAT) were obtained using PCR amplifications from a BAC of chromosome 8 (RP11–231D20) cloned into the pBLCAT3 enhancer vector (Promega® (Charbonnieres-les-bains, France)). All constructs were verified by sequencing. Two other t-PA promoter constructs (pt-PA0.4DR5-CAT and pt-PA2.4-DR5-CAT) were kindly provided by Frank Bulens [22]. The generation of mutated DR5 or Sp1 site into pt-PA0.4DR5-CAT construct was carried out using the GeneTailor™ Site-Directed Mutagenesis System (Invitrogen®). The mutagenesis primers designed for the mutations are reported in Table 1. The DR5 and Sp1 sites were, respectively, mutated from 5′-GGGTCACC CTGGGGTCA-3′ to 5′-GGTATTACGCACTAAAC-3′ and from 5′-A GCCCGCCCC-3′ to 5′-GATCGTTACT-3′. The mutated sequences were verified by DNA sequencing. Human RAR and RXR expression plasmids were kindly provided by Pierre Chambon (IGBMC, Strasbourg, France) [23, 24]. Plasmid pDR5-tk-CAT contains two copies of the RA-responsive element DR5 [25]. To normalize transfection efficiency, the pCH110 vector containing the β-galactosidase gene (driven by the cytomegalovirus [CMV] promoter) was cotransfected. SureSilencing™ RAR-β shRNA and negative control shRNA plasmids (KH00459N) containing neomycin resistance were purchased from SABiosciences® (Frederick, MD, USA).
Fig 2.
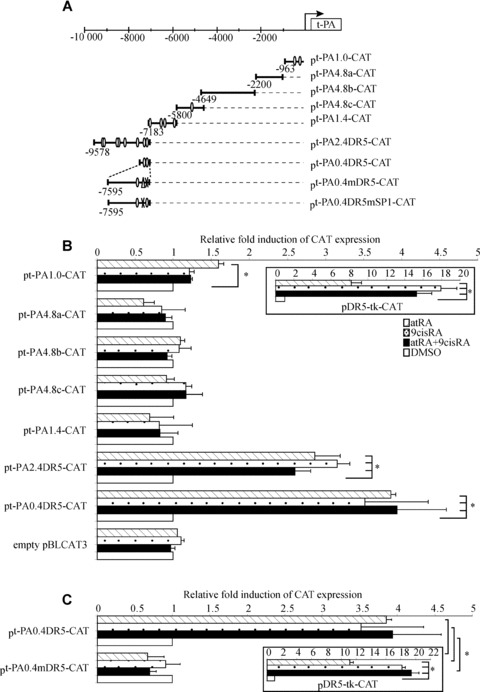
t-PA gene promoter contains a functional DR5 response element located at –7 kb. (A) Schematic representation of different constructs of the t-PA gene promoter. The t-PA promoter contains putative retinoid response elements (open circle) and a putative SP1 fixation site sequence (open triangle). (B) WISH cells were transfected with plasmid containing different lengths of the t-PA gene promoter in combination with the human RAR-α/RXR-α expression vectors pSG5-hRAR-α and pSVL-hRXR-α and then exposed to 10−6 M atRA and/or 9cisRA for 24 hrs. (C) Site-directed mutagenesis analysis of the putative DR5 sequence in the t-PA promoter. WISH cells were transiently transfected with wild-type pt-PA0.4DR5 or mutant pt-PA0.4mDR5 with the human RAR-α/RXR-α expression vectors exposed to 10−6 M atRA and/or 9cisRA for 24 hrs. Positive control was performed using a construct containing a double DR5 linked to thymidine kinase (boxed illustrations). Results are expressed as fold induction relative to the control treatment sample. Each value represents the mean ± S.D. of three separate transfections, each performed in duplicate. *P < 0.05.
Transfection of WISH cells
The amnion-derived WISH epithelial cells were trypsinized 16 hrs before transfection in 6-well plates. A total of 3 × 105 cells were transfected using GeneJammer with 1.45 μg of different t-PA constructs or positive control pDR5-tk-CAT plasmid and 0.25 μg of pCH110 β-galactosidase vector. After overnight incubation, the cells were treated for 24 hrs with retinoids (atRA and/or 9cisRA), using an optimized concentration for retinoid-driven WISH stimulation, as previously established [19], and/or LE135 RAR-β antagonist at 10−6 M [20]. For all the experiments, the maximal DMSO concentration to which the cells were exposed was <0.1%. Cell viability assays, using cells supernatants, were performed for each treatment (retinoids and DMSO) using XTT assays (Roche Diagnostics®). The average values of cells grown in regular medium were considered as 100% viability; no toxicity >10% was observed for retinoids or for DMSO treatment. At 48 hrs after transfection, the cells were harvested and lysed before performing CAT (Roche Diagnostics®) and β-galactosidase assays (Stratagene®) according to the manufacturer protocols.
Immunoprecipitation
Transfected WISH cells were washed twice with PBS and cell lysates were prepared by resuspension of the cells with gentle rocking in ice-cold IPH buffer (50 mM Tris pH8, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid [EDTA], 0.5% NP40 and protease inhibitors). The supernatants were incubated overnight with 3 μg of RAR-α (sc-551), RAR-β (sc-552), RAR-γ (sc-550) or RXR-α (sc-553) rabbit antibodies (Santa Cruz Biotechnology® (Santa Cruz, CA, USA)) and then with protein A/G plus agarose beads (Santa Cruz Biotechnology®) for 2 hrs. Negative controls were conducted in a similar manner using rabbit IgG. Agarose beads were then washed five times with ice-cold IPH buffer in 60 μl of 4× sample loading buffer. Total immunoprecipitated protein was loaded onto 10% SDS-PAGE gels, separated, and transferred onto PVDF membranes. The membranes were blocked with PBS-5% milk and incubated with Sp1 mouse antibody (sc-59, 1/200; Santa Cruz Biotechnology®). After washing, the membranes were stained with secondary donkey polyclonal HRP antimouse antibody (1/5000 in PBS-Tween 0.1%; Abcam®). Protein bands were visualized by chemiluminescent detection (ECLplus; GE Healthcare® (Clermont-Ferrand, France)).
Chromatin immunoprecipitation assay
Three 10-cm cell culture dishes of confluent WISH cells were used for each condition. The assay was conducted following the protocol described by Nelson et al.[26], with minor modifications concerning the Chelex-100-mediated DNA purification. A 151-bp region (XDR5t-PA) of the t-PA promoter and a 233-pb region (XDR5RAR-β) of the RAR-β promoter were amplified by specific primers, described in Table 1, on the different immunoprecipitated DNA obtained with antibodies raised against RAR-α, RAR-β, RAR-γ, RXR-α and Sp1 (3 μg) or IgG antibodies (negative control). PCR products were separated by electrophoresis on a 3% agarose gel. Band intensities were analysed (linear range of PCR amplification) by densitometry (Scion Image, Scion Corporation, Frederick, MD, USA) and normalized against input control (IC).
Statistical methods
Results expressed as means ± S.D. are an average of different experiments per condition. A comparison of the means was conducted by ANOVA analysis using StatView software (SAS Institute, Cary, NC, USA). For all the studies, the values were considered significantly different at P < 0.05.
Results
t-PA was expressed in amniotic membranes and WISH cells
As a first step to elucidate the role of t-PA in human foetal membranes, we examined t-PA expression in the placental environment. Using RT-PCR, we showed that t-PA mRNA was expressed not only in the placenta but also in foetal membranes (amnion and chorion) and WISH cells, with a PCR product of the expected size (243 bp; Fig. 1A). These data were confirmed by immunohistochemistry showing t-PA protein expression in amniotic and chorionic membranes as well as in WISH cells (Fig. 1B). Together, these findings validate the amnion-derived cell line WISH as a cellular model for studying the functional t-PA pathway in human amnion.
Fig 1.
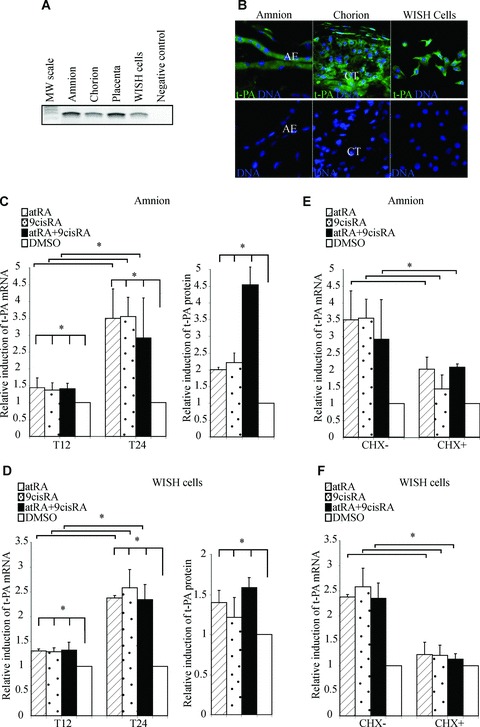
Retinoids up-regulate t-PA mRNA expression and proteins in human amniotic membrane and WISH cells. (A) First-strands cDNA prepared from the amnion, chorion, placenta and WISH cells were used to amplify t-PA mRNA. The observed RT-PCR product matched the size expected (243 pb). The PCR-negative control lane was performed without cDNA. (B) Representative immunofluorescence staining for t-PA protein on foetal membranes and WISH cells. t-PA protein was detected with an anti-t-PA polyclonal antibody recognized by an HRP-labelled secondary antibody and was revealed by a fluorescence amplification system (green). Nuclei were counterstained with DAPI (blue). AE, amniotic epithelium; CT, chorionic cytotrophoblasts. (C and D) The amnion (C) and WISH cells (D) were treated with 10−6 M atRA and/or 9cisRA for 12 and 24 hrs. Left panel: Total RNA was isolated and subjected to qPCR for t-PA transcript quantification. Right panel: t-PA protein concentrations were measured in cell extracts by ELISA, as described in the Materials and Methods section, and were normalized to total proteins extract concentrations. (E and F) The amnion (E) and WISH cells (F) were treated with or without cycloheximide (CHX), 10−6 M atRA and/or 9cisRA for 24 hrs. In all experiments, t-PA mRNA level was normalized to 36B4 and expressed as the level of induction relative to the control treatment (DMSO). First-strand cDNA was prepared from RNA and equal amounts were subjected to qRT-PCR analysis. Data reported are representative of three independent experiments, each performed in triplicate. Each bar gives means ± S.D. *P < 0.05.
t-PA expression was induced by retinoid treatment of amnion and WISH cells
To examine the role of retinoids in transcriptional regulation of t-PA, quantitative RT-PCR (qRT-PCR) analysis was performed after the amnion and WISH cells had been exposed to atRA and/or 9cisRA for 12 and 24 hrs (Fig. 1C and D/left panel). Both treatments of the amnion with 10−6 M of atRA and 9cisRA significantly enhanced t-PA mRNA levels. This induction could be only detected after a minimal exposure to retinoids for 12 hrs and reached an approximately three-fold increase after 24 hrs (Fig. 1C). Similar results were obtained on the WISH cell line, with an approximately 2.5-fold increase after 24 hrs (Fig. 1D). To confirm the retinoid actions on t-PA expression, we analysed their effects on t-PA protein expression in the amnion and WISH cells treated for 24 hrs with 10−6 M of atRA and/or 9cisRA. Retinoids significantly stimulated the expression of t-PA protein in the amnion (about four-fold) and WISH cells (about 1.6-fold; Fig. 1C and D/right panel).
Retinoid transactivation of t-PA is regulated by a two-step pathway in amnion
To determine whether retinoid-mediated induction of t-PA occurs through a direct regulation by atRA and 9cisRA, both the amnion and the WISH cells were treated by CHX, a well-established inhibitor of de novo protein synthesis (Fig. 1E and F). We showed that t-PA mRNA levels only increased about two-fold in the amnion compared with a 3.5-fold induction in the absence of CHX. In WISH cells, the t-PA induction was blocked by CHX (Fig. 1F). Using our cell model, these results suggest that the induction of t-PA was not only due to the direct action of retinoids but also dependent on combined mechanisms based on new protein synthesis.
Retinoid-induced t-PA promoter activity in WISH cells
In order to identify RA response element in the t-PA promoter, the transcriptional regulation of t-PA gene expression by retinoids was explored in transient transfection assays using various constructs of the t-PA promoter. Indeed, bioinformatics analysis using Genomatix (Le Pecq, France) software revealed that numerous putative retinoid response elements were present in the –10 kb t-PA promoter, including a putative DR5 located at –7 kb (Fig. 2A; open circle). Therefore, the t-PA promoter was cut into different portions containing or not containing putative retinoid response elements (Fig. 2A). A double DR5 linked to thymidine kinase was used as a positive control of retinoid transactivation of the different constructs, confirming retinoid activation (Fig. 2B). Transfection experiments with the −2200/−963 (pt-PA4.8a-CAT), −4649/−2200 (pt-PA4.8b-CAT), −5800/−4649 (pt-PA4.8c-CAT) and −7183/−5800 (pt-PA1.4-CAT) promoter fragments revealed that neither atRA or 9cisRA nor atRA and 9cisRA had any effect on CAT activity (Fig. 2B). In contrast, the −9578/−7183 promoter fragment (pt-PA2.4DR5-CAT) drove a significant 2.8-fold increase of reporter gene activity in response to atRA and/or 9cisRA, suggesting that this fragment contains one or more functional response elements. To precisely determine which is/are the functional retinoid response element(s) in this promoter, deletion constructs of the −9578/−7183 promoter fragment were run in similar experiments. Only the −7595/−7183 fragment (pt-PA0.4DR5-CAT) showed an approximately 3.8-fold induction of CAT activity in the presence of atRA and/or 9cisRA (Fig. 2B), strongly suggesting that this 400-pb fragment is sufficient to drive retinoid regulation of the t-PA gene. Moreover, bioinformatics analysis revealed that two putative retinoid response elements (DR5 and DR8) were present in this 400-pb fragment (contained in the pt-PA0.4DR5-CAT construct). As retinoid response elements are typically built of two consensus palindromic AGGTCA sequences separated by 5 pb [27], we performed site-directed mutagenesis of the DR5 response element found between −7212 and −7195 pb (pt-PA0.4mDR5-CAT). Transfection with pt-PA0.4mDR5-CAT showed that atRA and/or 9cisRA induction was completely abolished (Fig. 2C). There was no effect with mutation of DR8 response element (data not shown). These data demonstrated that DR5 located at −7212/−7195 pb mediates the activation of reporter gene expression by atRA and/or 9cisRA, suggesting that this DR5 response element controls t-PA gene regulation in WISH cells.
SP1 was involved in retinoid regulation of t-PA
In order to find transcription factors that could be further involved in the regulation of t-PA gene, we searched for other cis-acting elements that could be critical for retinoid regulation of the t-PA gene. Bioinformatics analysis revealed the presence of SP1 binding element on the 400-pb t-PA (pt-PA0.4DR5-CAT) construct (Fig. 2A; open triangle). Therefore, we hypothesized that RARs and RXRs might interact with SP1 in the regulation of t-PA expression in WISH cells. To confirm this hypothesis, we first checked SP1 expression in the extraembryonic environment (Fig. 3A). Using RT-PCR, we showed that SP1 was expressed in the amnion and WISH cells as in the placenta, which was used as a positive control [28]. To examine retinoid action on SP1 factor, the amnion and WISH cells were treated for 12 and 24 hrs by atRA and/or 9cisRA. qRT-PCR showed that atRA and/or 9cisRA had no effect on SP1 mRNA levels in the amnion (Fig. 3B) or in WISH cells (Fig. 3C). Moreover, WISH cells were also treated for 24 hrs with atRA and/or 9cisRA after transient transfections using pt-PA0.4DR5mSP1-CAT, in which we mutated the SP1 site located at −7248/−7238 pb. We showed that atRA and/or 9cisRA activation was abolished when this −7248/−7238 SP1 site was mutated (Fig. 3D), indicating that the −7248/−7238 SP1 binding motif is important for retinoid regulation of the t-PA gene. Previous studies have demonstrated a physical interaction between RARs/RXRs and SP1 during retinoid regulation of target genes [29]. In order to test this physical interaction during amniotic regulation of t-PA by RA, immunoprecipitation assays were performed using the 24-hr retinoid-treated WISH cells transfected by the human RARs, RXR-α and SP1 expression vectors. Figure 3(E) showed that SP1 was co-immunoprecipitated not only with RAR-α/RXR-α but also with RAR-β, suggesting that SP1 and RARs/RXR-α physically interact in our cell model.
Fig 3.
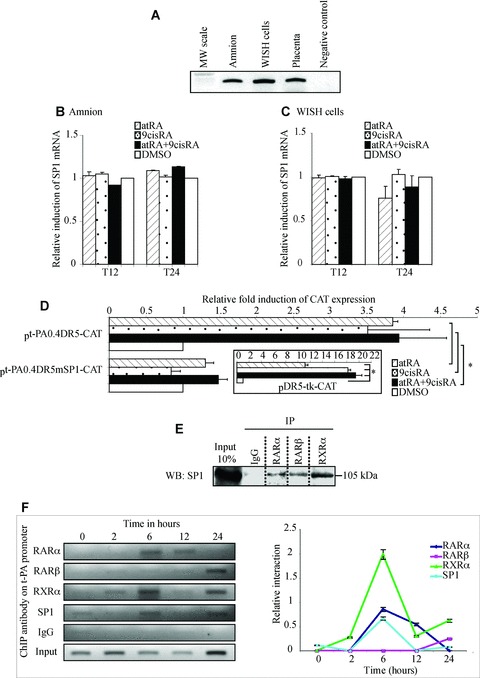
SP1 is necessary in retinoid-mediated regulation of the t-PA gene. (A) First-strand cDNA prepared from the amnion, WISH cells and placenta were used to amplify SP1 mRNA. Placenta cDNA sample was used as a positive control. The observed RT-PCR product matched the expected size (300 pb). (B and C) The amnion (B) and WISH cells (C) were treated with 10−6 M atRA and/or 9cisRA for 12 and 24 hrs. Total RNA was isolated and subjected to qPCR for SP1 transcript quantification. First-strand cDNA was prepared from RNA and equal amounts were subjected to qRT-PCR analysis. In all experiments, SP1 mRNA level was normalized to 36B4 and expressed as the level of induction relative to the control treatment (DMSO). Data reported are representative of three independent experiments, each performed in triplicate. Each bar gives mean ± S.D. (D) Site-directed mutagenesis analysis of the putative SP1 sequence in the t-PA promoter. WISH cells were transiently transfected with wild-type pt-PA0.4DR5-CAT or mutant pt-PA0.4mSP1-CAT with the human RAR-α/RXR-α expression vectors exposed to 10−6 M atRA and/or 9cisRA for 24 hrs. For each experiment, a positive control was performed using a construct containing a double DR5 linked to thymidine kinase (boxed illustration). Results are expressed as fold induction relative to the control treatment sample. Each value represents the mean ± S.D. of three separate transfections, each performed in duplicate. *P < 0.05. (E) WISH cells were exposed to 10−6 M atRA and 9cisRA for 24 hrs. Cell lysate proteins were immunoprecipitated with normal rabbit IgG or antibodies raised against RAR-α, RAR-β or RXR-α. Immunoprecipitated proteins were resolved by SDS-PAGE, transferred to PVDF membranes and probed with SP1 antibody. Input lane represents 10% of total protein extracts. IP, immunoprecipitation; WB, Western blot. (F) ChIP experiments were performed using WISH cells exposed to 10−6 M atRA and 9cisRA for different times (0, 2, 6, 12 and 24 hrs). Formaldehyde-fixed and sonicated lysates were subjected to immunoprecipitation using normal rabbit IgG or antibodies raised against RAR-α, RAR-β, RXR-α or SP1. Non-immunoprecipitated (input) and immunoprecipitated DNA were subjected to PCR using XDR5t-PA primers (151pb; Table 1) that amplify the t-PA promoter region containing DR5 response element and SP1 response element, as described in the Materials and Methods section. Enrichment of immunoprecipitated t-PA chromatin by RARs, RXR-α or SP1 antibodies was specific compared with ChIP performed using IgG control antibodies. Graph represents semi-quantitative relative interaction of RAR-α (lozenge), RAR-β (square), RXR-α (triangle) and SP1 (circle) compared with input control (obtained from n= 3 independent assays).
SP1, RARs and RXR-α bind to t-PA promoter
To examine SP1, RARs and RXR-α binding to their respective DNA response elements previously identified, ChIP assays were performed on WISH cells exposed to atRA and 9cisRA for different incubation periods (0, 2, 6, 12 and 24 hrs) using ChIP PCR primers designed to flank DR5 response element and SP1 binding motif located at −7 kb on t-PA promoter (Fig. 3F). The results revealed that RAR-α is able to bind DR5 after 6 and 12 hrs of retinoid treatment. Surprisingly, RAR-β (not naturally expressed in WISH cells) bound to DR5 t-PA promoter at 24 hrs. Because retinoid receptors are active as heterodimers, we tested the ability of RXR-α to bind to DR5 t-PA promoter. We showed that RXR-α binding to t-PA promoter was time dependent. Indeed, RXR-α weakly bound the DR5 site of t-PA promoter at 2 hrs and 12 hrs of retinoid treatment, whereas a higher enrichment of immunoprecipitated t-PA chromatin by RXR-α was detected at 6 and 24 hrs of retinoid exposure. Moreover, this experiment showed that SP1 binding is also time dependent, with high SP1 binding to its site at 6 hrs of retinoid treatment and weak binding at 24 hrs of retinoid exposure. Taken together, these results obtained on WISH cells suggest that retinoid regulation of t-PA gene is a dynamic process, with the first step (before 12 hrs of retinoid induction) involving RAR-α/RXR-α heterodimer and SP1 factor and the second step (around 24 hrs after retinoid treatment) involving RAR-β/RXR-α that replaces the earlier heterodimer, but always with SP1.
RAR-β expression increased after retinoid stimulation in amnion and WISH cells
To clarify the involvement of RAR-β in t-PA regulation, we first examined RAR-β expression after exposure of the amnion to atRA and/or 9cisRA for 12 and 24 hrs (Fig. 4A). qRT-PCR assays showed that RAR-β mRNA levels increased approximately two-fold in the presence of atRA or 9cisRA and increased three-fold with both atRA and 9cisRA at 12 hrs. This induction was also maintained after 24 hrs in the presence of retinoids. Similar experiments were performed in WISH cells treated with atRA and 9cisRA for different incubation times (0, 2, 6, 12 and 24 hrs; Fig. 4B). We confirmed that RAR-β was not expressed at T0 (e.g. in the absence of retinoids), in accordance with our previous studies [19]. The atRA- and 9cisRA-driven induction of RAR-β mRNA levels was time dependent in WISH cells. RAR-β began to be expressed after 2 hrs of exposure with atRA and 9cisRA. In our cell model, this expression increased by 1.3-fold at 6 hrs, 1.8-fold at 12 hrs and approximately 2.5-fold at 24 hrs.
Fig 4.
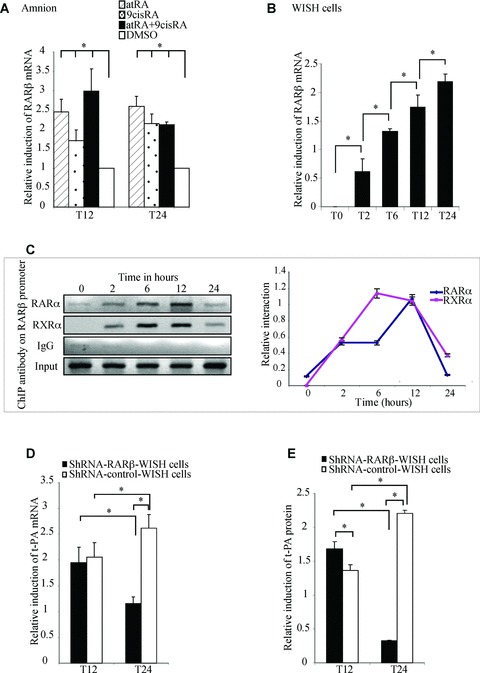
shRNA RAR-β strongly abolished the second-step retinoid-mediated induction of t-PA gene in WISH cells. (A) The amnion was treated with 10−6 M atRA and/or 9cisRA for 12 and 24 hrs. Total RNA was isolated and subjected to qPCR for RAR-β transcript quantification. First-strand cDNA was prepared from RNA and equal amounts were subjected to qRT-PCR analysis. (B) WISH cells were treated with 10−6 M atRA and 9cisRA for different periods of retinoid treatment (0, 2, 6, 12 and 24 hrs). Total RNA was isolated and subjected to qPCR for RAR-β transcript quantification. First-strand cDNA was prepared from RNA and equal amounts were subjected to qRT-PCR analysis. In all experiments, the level of RAR-β mRNA was normalized to 36B4 and expressed as the level of induction relative to the control treatment (DMSO). Data reported are representative of three independent experiments, each performed in triplicate. Each bar gives the mean ± S.D. *P < 0.05. (C) ChIP experiments were performed using WISH cells exposed to 10−6 M atRA and 9cisRA for different times (0, 2, 6, 12 and 24 hrs). Formaldehyde-fixed and sonicated lysates were subjected to immunoprecipitation using normal rabbit IgG or antibodies raised against RAR-α or RXR-α. Non-immunoprecipitated (input) and immunoprecipitated DNA were subjected to PCR using XDR5RAR-β primers (233 pb; Table 1) that amplify the RAR-β promoter region containing DR5 response element, as described in the Materials and Methods section. Interactions of proteins with DNA are specific, since there was no enrichment of immunoprecipi tated RAR-β promoter with IgG antibody compared with RAR-α and RXR-α antibodies. Graph represents semi-quantitative relative interaction of RAR-α (lozenge) and RXR-α (square) compared with input control. (D and E) WISH cells stably transfected with shRNA RAR-β (shRNA-RAR-β-WISH, black bar) or control shRNA (shRNA-control-WISH, white bar) were treated with 10−6 M atRA and 9cisRA for 12 and 24 hrs. Total RNA was isolated and the expression of t-PA was analysed by quantitative RT-PCR. The level of t-PA mRNA was normalized to 36B4 and expressed as the level of induction relative to the control treatment (DMSO). t-PA protein concentrations were quantified by ELISA assay, as described in the Materials and Methods section, and were normalized to total protein extract concentrations. Data reported are representative of three independent experiments. Each bar gives the mean ± S.D. *P < 0.05.
RAR-α is able to interact with the DR5 site of RAR-β promoter in WISH cells
As been previously described, a DR5-type response element is present in the RAR-β promoter sequence [30]. To verify the ability of RAR-α and RXR-α to interact with RAR-β promoter in the amniotic environment, ChIP experiments were performed on WISH cells treated with retinoids at different times using primers flanking the DR5 site of the RAR-β promoter (Fig. 4C). The results showed that RAR-α and RXR-α were able to bind RAR-β DR5 at 2, 6, 12 and 24 hrs after retinoid treatment. RAR-α binding appeared stronger at 6 and 12 hrs. No interaction could be detected for RAR-β and RAR-γ (data not shown). These results suggest that RAR-α/RXR-α heterodimers are involved in RAR-β transactivation by retinoids in the amniotic (WISH cells) environment.
RAR-β is necessary for retinoid-mediated regulation of t-PA in WISH cells
To confirm the role of RAR-β in t-PA regulation by retinoids, WISH cells stably transfected for shRNA RAR-β (shRNA-RAR-β-WISH) were established and treated with atRA and 9cisRA for 12 and 24 hrs. We first verified RAR-β extinction in terms of mRNA and proteins. No RAR-β mRNA was present at 12 hrs in shRNA-RAR-β-WISH cells, and RAR-β mRNA levels decreased by approximately 70% at 24 hrs compared with shRNA-control-WISH cells. At the protein level, shRNA-RAR-β-WISH cells showed a similar decrease of RAR-β proteins at 24 hrs (data not shown). t-PA mRNA and protein levels were then quantified in shRNA-RAR-β-WISH cells retinoid-treated for 12 and 24 hrs. As shown in Fig. 4(D), the induction of t-PA mRNA levels decreased by 56% in shRNA-RAR-β-WISH cells after 24 hrs of retinoid treatment compared with shRNA-control-WISH cells. In term of proteins (Fig. 4E), the absence of RAR-β had a significant weak effect on t-PA levels after 12 hrs of retinoid treatment, whereas t-PA induction had decreased by approximately 85% after 24 hrs of retinoid stimulation. This result is in accordance with our previous results indicating that RAR-β protein only interacts with t-PA promoter around 24 hrs after retinoid-mediated induction (Fig. 3F). To deeply analyse the involvement of RAR-β in t-PA retinoid regulation, shRNA-RAR-β-WISH and shRNA-control-WISH cells were transiently transfected with pt-PA0.4DR5, treated by atRA and/or 9cisRA for 24 hrs and submitted to CAT reporter assay. The results revealed that CAT induction was lower in cells expressing shRNA-RAR-β-WISH as well as in the presence of atRA and both atRA and 9cisRA compared with shRNA-control-WISH cells after the 24-hr retinoid treatment (data not shown).
RAR-β antagonist inhibits retinoid-mediated induction of t-PA in WISH cells
In order to confirm by a pharmacological approach that the retinoid-mediated induction of t-PA expression occurs through RAR-β, WISH cells were treated with 10−6 M RAR-β-selective antagonist LE135 [10] simultaneously with 10−6 M atRA and 9cisRA and submitted to qRT-PCR and t-PA protein assays. As shown in Fig. 5(A), atRA and 9cisRA induction of t-PA mRNA was significantly blocked by RAR-β antagonist after the 24-hr retinoid exposure. Retinoid-mediated induction of t-PA mRNA levels was decreased by approximately 70% after atRA and 9cisRA treatment in the presence of LE135. Similarly, LE135 blocked t-PA protein induction by retinoids, which had decreased by 81% at 24 hrs (Fig. 5B). WISH cells were transfected with pt-PA0.4DR5-CAT and treated with RAR-β antagonist, atRA and 9cisRA for 24 hrs. In our cell model, CAT reporter analysis assay showed that retinoid-mediated t-PA expression was similarly inhibited in the presence of RAR-β antagonist (data not shown).
Fig 5.
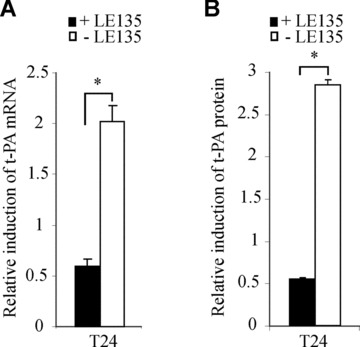
RAR-β antagonist LE135 blocked the retinoid-mediated induction of t-PA gene in WISH cells. (A and B) WISH cells were treated with 10−6 M LE135 and 10−6 M atRA. (A) Total RNA was isolated and the expression of t-PA was analysed by quantitative RT-PCR. The level of t-PA mRNA was normalized to 36B4 and expressed as the level of induction relative to the control treatment (DMSO). (B) t-PA protein concentrations were quantified by ELISA assay, as described in the Materials and Methods section, and were normalized to total protein extract concentrations. Data reported are representative of three independent experiments. Each bar gives the mean ± S.D. *P < 0.05.
Discussion
The amnion presents important adaptive structural and biochemical properties that enable it to follow gestational changes in the foetus throughout pregnancy and to participate at the right time in parturition. These gestational adaptations are strongly based on ECM proteins, whose precise organization and turnover have to be finely regulated by complex network of molecular signalling. A disorganization of these molecular regulations combined with premature disruption of the amnion can lead to an obstetrical pathology called pre-term pre-labour rupture of foetal membranes. The t-PA is one of the major proteins involved in the ECM degradation of human foetal membranes. In order to provide new clues for a better understanding of the physiopathology of foetal membranes, the precise mechanism of t-PA gene regulation has to be clarified and, more particularly, specified in terms of its relationships with retinoids, the active derivatives of vitamin A clearly implicated in embryonic and extraembryonic development [18].
We demonstrated retinoid-mediated induction of t-PA gene expression in the amnion and amniotic WISH cells (suspected to have a HeLa contamination). This is the first gene described as being regulated by retinoids in the amniotic environment. Furthermore, this regulation involved an additional partner SP1, forming a trimeric complex with RAR/RXR heterodimers. This complex was able to interact with the DR5 retinoid response element and an SP1 binding motif located at −7 kb of the t-PA transcription start site. We established that this t-PA induction occurs through two different successive steps and propose a schematic regulation (Fig. 6). In the first step (before and up to the 12-hr retinoid stimulation), retinoid-mediated induction allowed RAR-α/RXR-α heterodimers to bind to the DR5 response element of RAR-β gene and to the DR5 site of t-PA gene to stimulate both gene transcriptions (Fig. 6A). In the second step (Fig. 6B), RAR-β/ RXR-α heterodimers interact with the same DR5 response element of the t-PA gene, allowing a longer induction of t-PA expression. By the establishment of such a molecular pathway, our work is also the first report identifying t-PA gene regulation in the extraembryonic environment, and more particularly the amnion, and demonstrating the RAR-β induction involvement in a cellular environment, in which RAR-β is not basically expressed at detectable levels [19].
Fig 6.
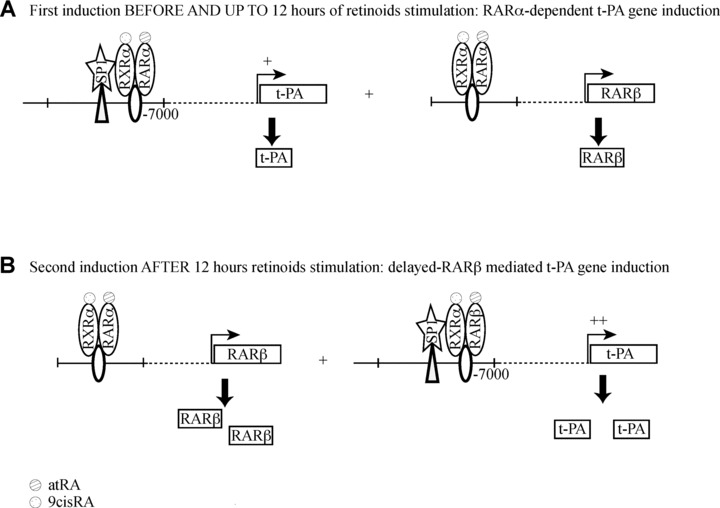
Proposed model for t-PA regulation by retinoids in human amnion. (A) First-step regulation of t-PA by a RAR-α-dependent pathway. Before and up to 12 hrs, retinoids simultaneously stimulate t-PA and RAR-β expression through RAR-α signalling. (B) Second-step regulation of t-PA by a RAR-β-delayed pathway. After 12 hrs, RAR-β is able to induce a higher t-PA expression than in the first step.
The initial absence and subsequent induction of RAR-β could be considered as having an important significance in the physiology of foetal membranes. Indeed, many structural changes occur around the time of delivery and, in particular, at the rupture of foetal membranes. The molecular pathways regulating the programmed degradation leading to the rupture of these membranes are still poorly understood. The induction of RAR-β (potentially boosted by a positive autoregulatory loop [30]) could be a key point of this regulation. Indeed, during parturition, t-PA is expressed at a basal level (Fig. 6), leading to a weak turnover of the ECM and a global stability of foetal membranes. At the point where the membranes rupture, RAR-β could be the major signal involved in the process, weakening these membranes by up-regulating t-PA production and accelerating ECM degradation. This hypothesis is supported by data from Bogic et al., who demonstrated that t-PA expression increases in pre-term delivery [10], and from Jenkins et al., who showed that plasminogen levels are proportional to the scale of amniotic epithelial cell degeneration after pre-term pre-labour membrane rupture [31].
In this kind of developmental cascade, it is important to identify the molecular mechanisms of RAR-β activation. RAR-β activity could be reinforced by a time-specific phosphorylation. Indeed, it was previously established that RAR-β could be phosphorylated on tyrosine residues [32]. Treating HUVEC with RA increases t-PA production through a pathway that involves protein kinases [33]. The activation of protein kinase C was also recently reported to induce t-PA expression in human astrocytes [13]. The explanation for RAR-β induction could also be linked to the modifications in specific homeostasis and the metabolism of retinoids in foetal membranes at delivery. Indeed, we and Lachili’s group demonstrated that vitamin A plasma concentrations significantly decreased at delivery compared with non-pregnant control groups [34, 35]. It was also established that at delivery, maternal β-carotene and vitamin A plasma concentrations were significantly higher than foetal cord blood concentrations [36], suggesting a possible uptake of retinoid stocks to allow the physiological phenomenon linked to parturition. We could hypothesize that this metabolic context specific to parturition could produce sufficient quantities of retinoids to induce amniotic RAR-β expression, which could not occur at other times of pregnancy.
Our work identified the importance of SP1 transcription factor in the retinoid-mediated regulation of t-PA in amniotic cells, showing that SP1 and RAR/RXR heterodimers cooperate and interact to transcriptionally activate amniotic t-PA gene. An increasing body of data suggests that retinoid responsiveness not only occurs via RAR/RXR heterodimers but also via cross-talk with other transcription factor pathways or, more particularly, via interaction with SP1. For example, RARs physically interact with SP1 in retinoid-mediated interleukin-1β (IL-1β) gene regulation [29], and an identical mechanism has been also described for urokinase (UK) gene regulation [37]. Others studies have shown that RARs functionally interact with SP1 in the retinoid-mediated regulation of transglutaminase gene [38], transforming growth factor-β1 (TGF-β1) [39] and retinol binding protein (RBP) gene [40]. The involvement of SP1 in t-PA regulation could be linked to the fact that a polymorphism located in the SP1 binding motif on the t-PA promoter alters t-PA response to retinoids [41]. In our study, the amnion explants and WISH cells did not show this polymorphism on the t-PA gene at the SP1 site, presenting a normal response to retinoid stimulation. We could hypothesize that women presenting this polymorphism could present a reduced response to retinoid stimulation and be less sensitive to this signal, which is necessary for membrane ruptures.
At parturition, the degradation of ECM proteins in amniotic membranes mainly involves t-PA, which could be also helped by other members of the t-PA cascade such as the urokinase plasminogen activator (u-PA) or the MMPs (MMP2 and MMP9). We confirmed the role of retinoids in the regulation of the t-PA system by establishing the activation of u-PA transcription (approximately three-fold) and MMP2 and MMP9 (approximately two-fold, preliminary data). These data were in agreement with the results from previous studies performed in other cellular models [37, 42, 43] and strengthened the global effects of retinoids on the t-PA system in the amniotic environment.
In conclusion, our report is the first study demonstrating, in the amniotic environment, a two-step mechanism of retinoid-mediated t-PA regulation involving a RAR-α-dependent regulation in the first step and a RAR-β-delayed regulation in the second step of retinoid stimulation. In terms of physiopathology, the increase of knowledge concerning the t-PA gene regulation by the retinoids was an essential step towards a better understanding of the pre-term labour rupture of foetal membranes, in which RAR-β expression levels could therefore be compared with those of labour and term rupture. This would then make it possible to develop new strategies based on retinoids for the prevention and treatment of this deleterious phenomenon during human pregnancy.
Acknowledgments
We thank A.T.T® (Auvergne Traduction Technique) for proofreading the manuscript. We thank Dr. Pierre Chambon for generously donating human RAR/RXR expression plasmids and Dr. Bulens for providing the t-PA promoter gene constructs. V.B was supported by a grant from the Ministère de l’Education de la Recherche et de la Technologie (MERT). G.M. and V.S. received financial support from INSERM grants (‘Poste Accueil’ and ‘Contrat Interface’, respectively). D.G. received financial support from INSERM grants (‘Contrat Interface’) and from the Société Française de Médecine Périnatale and the Collège National des Gynécologues et Obstétriciens Français.
References
- 1.Niknejad H, Peirovi H, Jorjani M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 2.Bernirschke K, Kaufman P. Pathology of the human placenta. 4th edition. New York: Springer-Verlag; 1995. [Google Scholar]
- 3.Malak TM, Ockleford CD, Bell SC, et al. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14:385–406. doi: 10.1016/s0143-4004(05)80460-6. [DOI] [PubMed] [Google Scholar]
- 4.Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19:1–11. doi: 10.1016/s0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 5.Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–70. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 7.DeClerck YA, Laug WE. Cooperation between matrix metalloproteinases and the plasminogen activator-plasmin system in tumor progression. Enzyme Protein. 1996;49:72–84. doi: 10.1159/000468617. [DOI] [PubMed] [Google Scholar]
- 8.Lijnen HR, Silence J, Lemmens G, et al. Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb Haemost. 1998;79:1171–6. [PubMed] [Google Scholar]
- 9.Bryant-Greenwood GD, Schwabe C. Human relaxins: chemistry and biology. Endocr Rev. 1994;15:5–26. doi: 10.1210/edrv-15-1-5. [DOI] [PubMed] [Google Scholar]
- 10.Bogic LV, Ohira RH, Yamamoto SY, et al. Tissue plasminogen activator and its receptor in the human amnion, chorion, and decidua at preterm and term. Biol Reprod. 1999;60:1006–12. doi: 10.1095/biolreprod60.4.1006. [DOI] [PubMed] [Google Scholar]
- 11.Kooistra T, Lansink M, Arts J, et al. Involvement of retinoic acid receptor alpha in the stimulation of tissue-type plasminogen-activator gene expression in human endothelial cells. Eur J Biochem. 1995;232:425–32. [PubMed] [Google Scholar]
- 12.Montemurro P, Barbuti G, Conese M, et al. Retinoic acid stimulates plasminogen activator inhibitor 2 production by blood mononuclear cells and inhibits urokinase-induced extracellular proteolysis. Br J Haematol. 1999;107:294–9. doi: 10.1046/j.1365-2141.1999.01698.x. [DOI] [PubMed] [Google Scholar]
- 13.Hultman K, Tjarnlund-Wolf A, Fish RJ, et al. Retinoids and activation of PKC induce tissue-type plasminogen activator expression and storage in human astrocytes. J Thromb Haemost. 2008;6:1796–803. doi: 10.1111/j.1538-7836.2008.03084.x. [DOI] [PubMed] [Google Scholar]
- 14.Rickles RJ, Darrow AL, Strickland S. Differentiation-responsive elements in the 5’ region of the mouse tissue plasminogen activator gene confer two-stage regulation by retinoic acid and cyclic AMP in teratocarcinoma cells. Mol Cell Biol. 1989;9:1691–704. doi: 10.1128/mcb.9.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merchiers P, Bulens F, De Vriese A, et al. Involvement of Sp1 in basal and retinoic acid induced transcription of the human tissue-type plasminogen activator gene. FEBS Lett. 1999;456:149–54. doi: 10.1016/s0014-5793(99)00942-4. [DOI] [PubMed] [Google Scholar]
- 16.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–53. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 17.Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–25. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 18.Marceau G, Gallot D, Lemery D, et al. Metabolism of retinol during mammalian placental and embryonic development. Vitam Horm. 2007;75:97–115. doi: 10.1016/S0083-6729(06)75004-X. [DOI] [PubMed] [Google Scholar]
- 19.Marceau G, Gallot D, Borel V, et al. Molecular and metabolic retinoid pathways in human amniotic membranes. Biochem Biophys Res Commun. 2006;346:1207–16. doi: 10.1016/j.bbrc.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Hashimoto Y, Agadir A, et al. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem. 1999;274:15360–6. doi: 10.1074/jbc.274.22.15360. [DOI] [PubMed] [Google Scholar]
- 21.Han YM, Romero R, Kim JS, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–61. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulens F, Ibanez-Tallon I, Van Acker P, et al. Retinoic acid induction of human tissue-type plasminogen activator gene expression via a direct repeat element (DR5) located at –7 kilobases. J Biol Chem. 1995;270:7167–75. doi: 10.1074/jbc.270.13.7167. [DOI] [PubMed] [Google Scholar]
- 23.Brand N, Petkovich M, Krust A, et al. Identification of a second human retinoic acid receptor. Nature. 1988;332:850–3. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- 24.Elder JT, Astrom A, Pettersson U, et al. Differential regulation of retinoic acid receptors and binding proteins in human skin. J Invest Dermatol. 1992;98:673–9. doi: 10.1111/1523-1747.ep12499896. [DOI] [PubMed] [Google Scholar]
- 25.Mader S, Chen JY, Chen Z, et al. The patterns of binding of RAR, RXR and TR homo- and heterodimers to direct repeats are dictated by the binding specificites of the DNA binding domains. EMBO J. 1993;12:5029–41. doi: 10.1002/j.1460-2075.1993.tb06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc. 2006;1:179–85. doi: 10.1038/nprot.2006.27. [DOI] [PubMed] [Google Scholar]
- 27.Ross SA, McCaffery PJ, Drager UC, et al. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–54. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 28.Lania L, Majello B, De Luca P. Transcriptional regulation by the Sp family proteins. Int J Biochem Cell Biol. 1997;29:1313–23. doi: 10.1016/s1357-2725(97)00094-0. [DOI] [PubMed] [Google Scholar]
- 29.Husmann M, Dragneva Y, Romahn E, et al. Nuclear receptors modulate the interaction of Sp1 and GC-rich DNA via ternary complex formation. Biochem J. 2000;352:763–72. [PMC free article] [PubMed] [Google Scholar]
- 30.de The H, Vivanco-Ruiz MM, Tiollais P, et al. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–80. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins DM, O’Neill M, Mattar M, et al. Degenerative changes and detection of plasminogen in fetal membranes that rupture prematurely. Br J Obstet Gynaecol. 1983;90:841–6. doi: 10.1111/j.1471-0528.1983.tb09325.x. [DOI] [PubMed] [Google Scholar]
- 32.Rochette-Egly C, Gaub MP, Lutz Y, et al. Retinoic acid receptor-beta: immunodetection and phosphorylation on tyrosine residues. Mol Endocrinol. 1992;6:2197–209. doi: 10.1210/mend.6.12.1283441. [DOI] [PubMed] [Google Scholar]
- 33.Thompson EA, Nelles L, Collen D. Effect of retinoic acid on the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in human endothelial cells. Eur J Biochem. 1991;201:627–32. doi: 10.1111/j.1432-1033.1991.tb16323.x. [DOI] [PubMed] [Google Scholar]
- 34.Lachili B, Faure H, Smail A, et al. Plasma vitamin A, E, and beta-carotene levels in adult post-partum Algerian women. Int J Vitam Nutr Res. 1999;69:239–42. doi: 10.1024/0300-9831.69.4.239. [DOI] [PubMed] [Google Scholar]
- 35.Sapin V, Alexandre MC, Chaib S, et al. Effect of vitamin A status at the end of term pregnancy on the saturation of retinol binding protein with retinol. Am J Clin Nutr. 2000;71:537–43. doi: 10.1093/ajcn/71.2.537. [DOI] [PubMed] [Google Scholar]
- 36.Scaife AR, McNeill G, Campbell DM, et al. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br J Nutr. 2006;95:771–8. doi: 10.1079/bjn20051718. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Shimada J, Shudo K, et al. Physical interaction between retinoic acid receptor and Sp1: mechanism for induction of urokinase by retinoic acid. Blood. 1999;93:4264–76. [PubMed] [Google Scholar]
- 38.Lu S, Saydak M, Gentile V, et al. Isolation and characterization of the human tissue transglutaminase gene promoter. J Biol Chem. 1995;270:9748–56. doi: 10.1074/jbc.270.17.9748. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, Ratziu V, Choi SG, et al. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273:33750–8. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 40.Panariello L, Quadro L, Trematerra S, et al. Identification of a novel retinoic acid response element in the promoter region of the retinol-binding protein gene. J Biol Chem. 1996;271:25524–32. doi: 10.1074/jbc.271.41.25524. [DOI] [PubMed] [Google Scholar]
- 41.Wolf AT, Medcalf RL, Jern C. The t-PA -7351C>T enhancer polymorphism decreases Sp1 and Sp3 protein binding affinity and transcriptional responsiveness to retinoic acid. Blood. 2005;105:1060–7. doi: 10.1182/blood-2003-12-4383. [DOI] [PubMed] [Google Scholar]
- 42.Dalmolin RJ, Zanotto-Filho A, De Oliveira RB, et al. Retinol and retinoic acid increase MMP-2 activity by different pathways in cultured Sertoli cells. Free Radic Res. 2007;41:1338–47. doi: 10.1080/10715760701717427. [DOI] [PubMed] [Google Scholar]
- 43.Zaragoza R, Gimeno A, Miralles VJ, et al. Retinoids induce MMP-9 expression through RARalpha during mammary gland remodeling. Am J Physiol Endocrinol Metab. 2007;292:E1140–8. doi: 10.1152/ajpendo.00463.2006. [DOI] [PubMed] [Google Scholar]


