Abstract
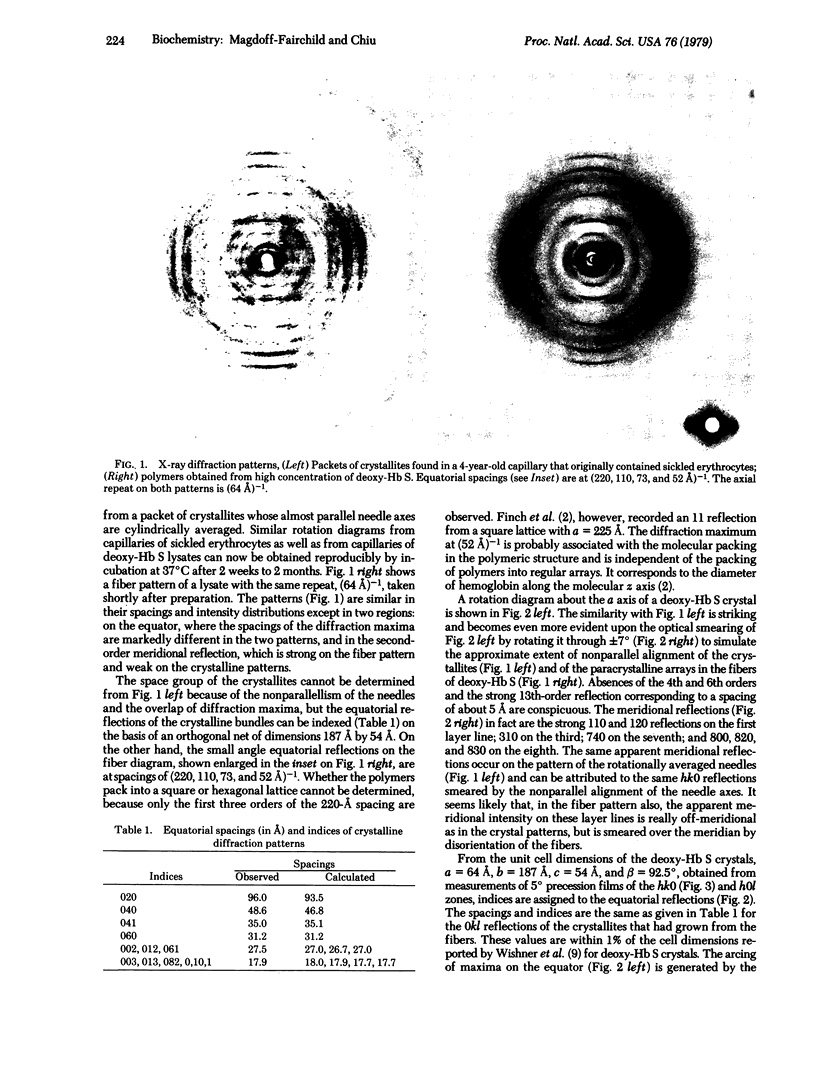
Paracrystalline fibers of deoxygenated sickle hemoglobin in erythrocytes or concentrated solutions exhibit a phase transformation to a fully crystalline state. X-ray diffraction patterns of the fiber and crystallites are similar except in two respects: the equatorial spacings of the fibers suggest that they pack into a square lattice with a = 220 A, whereas those of the crystals can be indexed on the basis of a net of 187 A by 54 A, and the second-order near-meridional reflections are strong on the fiber pattern but weak on that of the crystallites. The crystallites are isomorphous with single crystals grown in polyethylene glycol solution at pH 4.5 whole structure has been determined at near-atomic resolution (Wishner, B.C., Ward, K.B. Lattmen, E.E. & Lowve, W.E. (1975) J. Mol. Biol. 98, 179-194). Double filaments of molecules with an axial repeat of 64 A comprise the basic unit of both the crystal and fiber structures. Each filament of the pair is translated with respect to its neighbor by half a molecular diameter along the fiber axis. The two filaments are held together by contacts made by Val 6beta in the molecules of one strand with hydrophobic side chains of the molecule in the neighboring strand. This interaction is probably the cause of the aggregation of filaments into fibers that leads to the sickling of erythrocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dykes G., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the fibres of sickle cell haemoglobin. Nature. 1978 Apr 6;272(5653):506–510. doi: 10.1038/272506a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frier J. A., Perutz M. F. Structure of human foetal deoxyhaemoglobin. J Mol Biol. 1977 May 5;112(1):97–112. doi: 10.1016/s0022-2836(77)80158-7. [DOI] [PubMed] [Google Scholar]
- Josephs R., Jarosch H. S., Edelstein S. J. Polymorphism of sickle cell hemoglobin fibers. J Mol Biol. 1976 Apr 15;102(3):409–426. doi: 10.1016/0022-2836(76)90324-7. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Poillon W. N., Li T., Bertles J. F. Thermodynamic studies of polymerization of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1976 Apr;73(4):990–994. doi: 10.1073/pnas.73.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- Messana C., Cerdonio M., Shenkin P., Noble R. W., Fermi G., Perutz R. N., Perutz M. F. Influence of quaternary structure of the globin on thermal spin equilibria in different methemoglobin derivatives. Biochemistry. 1978 Aug 22;17(17):3652–3662. doi: 10.1021/bi00610a035. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Ohtsuki M., White S. L., Zeitler E., Wellems T. E., Fuller S. D., Zwick M., Makinen M. W., Sigler P. B. Electron microscopy of fibers and discs of hemoglobin S having sixfold symmetry. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5538–5542. doi: 10.1073/pnas.74.12.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]