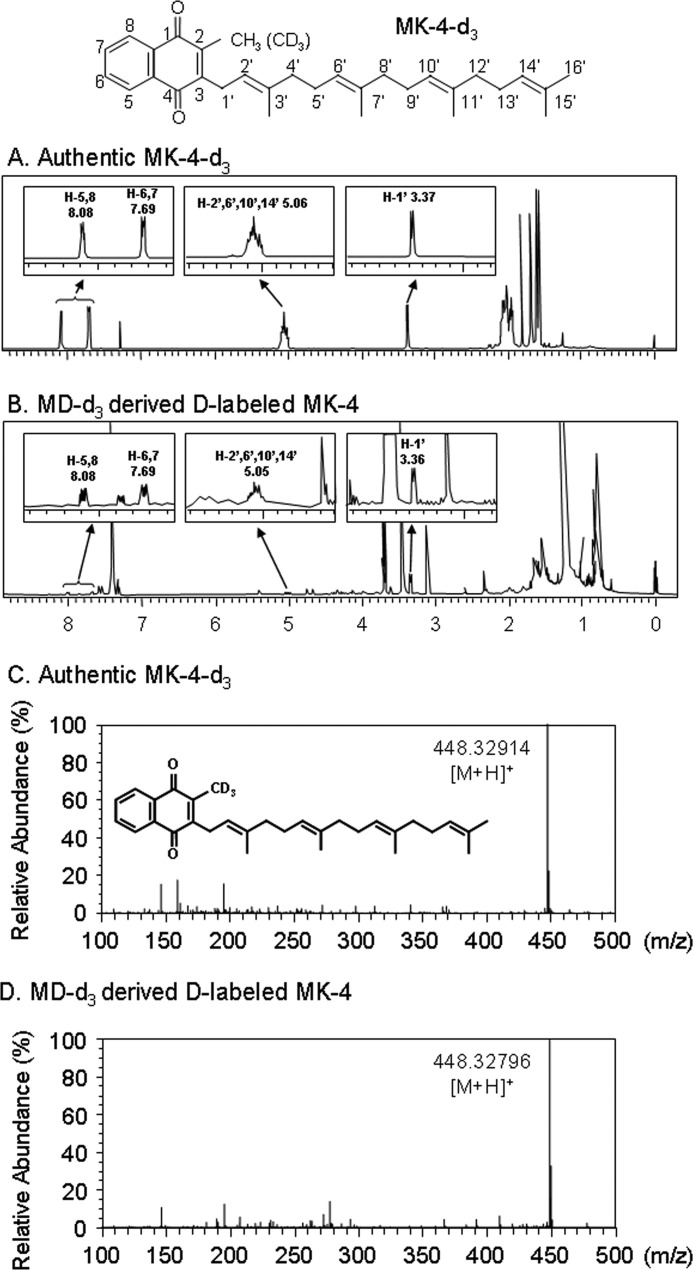
FIGURE 6.
1H NMR analysis and HR-MS analysis of menadione labeled with deuterium at the 2-methyl group (MD-d3)-derived MK-4 generated by the incubation of MD-d3 with rhUBIAD1 in the presence of GGPP. MD-d3 (2 × 10−4 m) was incubated at 37 °C for 3 h with rhUBIAD1 (10 μg as protein weight of the microsomes prepared from UBIAD1 baculovirus-infected Sf9 cells) in the presence of GGPP (2 × 10−4 m) and DTT (1 mm) in 100 mm Tris-HCl buffer (pH 7.8). After incubation, the lipids were extracted with 10 ml of acetone/ethanol (9:1) solution. The extract was centrifuged at 2,500 rpm for 5 min at 4 °C, and the supernatant (2.5 ml) was evaporated under reduced pressure. This procedure was repeated 1,200 times. All the residues were then redissolved in 2 ml of hexane, evaporated under reduced pressure, and then dissolved in 60 μl of CDCl3 to yield the resulting samples. An aliquot of the solution was used for 1H NMR analysis. A and B, 1H NMR spectra of authentic MK-4-d3 and MD-d3-derived MK-4, respectively. The number and letter H in each spectrum refer to the chemical shift (ppm) and the respective position of the proton in the 2-methyl-1,4-naphthoquinone ring or the side chain of MK-4-d3. After 1H NMR analysis of MD-d3-derived MK-4, the sample solution was evaporated under reduced pressure, dissolved in 100 μl of methanol, and used for HR-MS analysis. C and D, HR-MS spectra of authentic MK-4-d3 and MD-d3-derived MK-4, respectively. MS was performed on an Exactive Orbitrap mass spectrometer (Thermo Scientific) operating in the positive ion mode as described under “Experimental Procedures.”