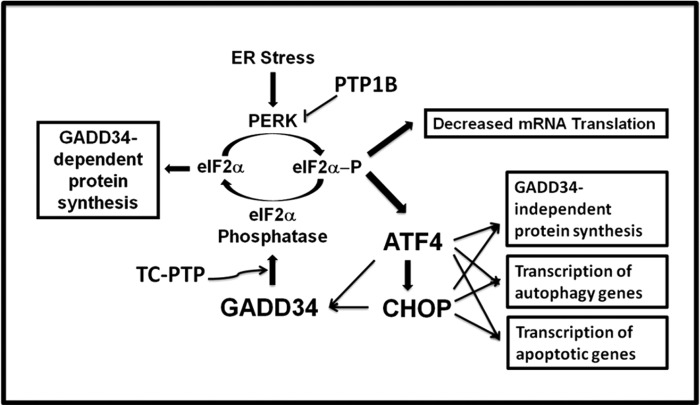
FIGURE 10.
Protein tyrosine phosphatases in the control of eIF2α phosphorylation and cell fate. ER stress has been extensively documented to activate PERK, which phosphorylates eIF2α to inhibit mRNA translation and general protein synthesis. However, translation of the mRNA encoding the transcription factor, ATF4, is enhanced following eIF2α phosphorylation. ATF4 then promotes the transcription of the gene, encoding CHOP, and ATF4 and CHOP collaborate to promote the expression of GADD34 as well as many autophagy genes (35). Finally, GADD34 assembles an eIF2α phosphatase restores general protein synthesis. ATF4 and CHOP also collaborate to turn on numerous genes that may promote both GADD34-dependent and GADD34-independent protein synthesis (23, 24) and the ensuing oxidative stress plays a key role in triggering programmed cell death (24). CHOP, possibly in conjunction with ATF4, enhances the transcription of numerous proapoptotic genes (36). Thus, the current evidence suggests that both the phosphorylation and dephosphorylation of eIF2α can induce apoptosis (36). In this context, the rapid turnover of mRNAs and proteins encoding ATF4, CHOP, and GADD34, is likely to be a critical factor in determining cell fate (9). While prior work suggested that PTP1B antagonized PERK activity, the current work highlighted the ability of TC-PTP to control GADD34 protein stability, potentially enhancing eIF2α dephosphorylation. Thus, the coordinate regulation of these PTPases by ER stress may fine-tune eIF2α phosphorylation and downstream signaling that determines the cell's decision to survive or die.