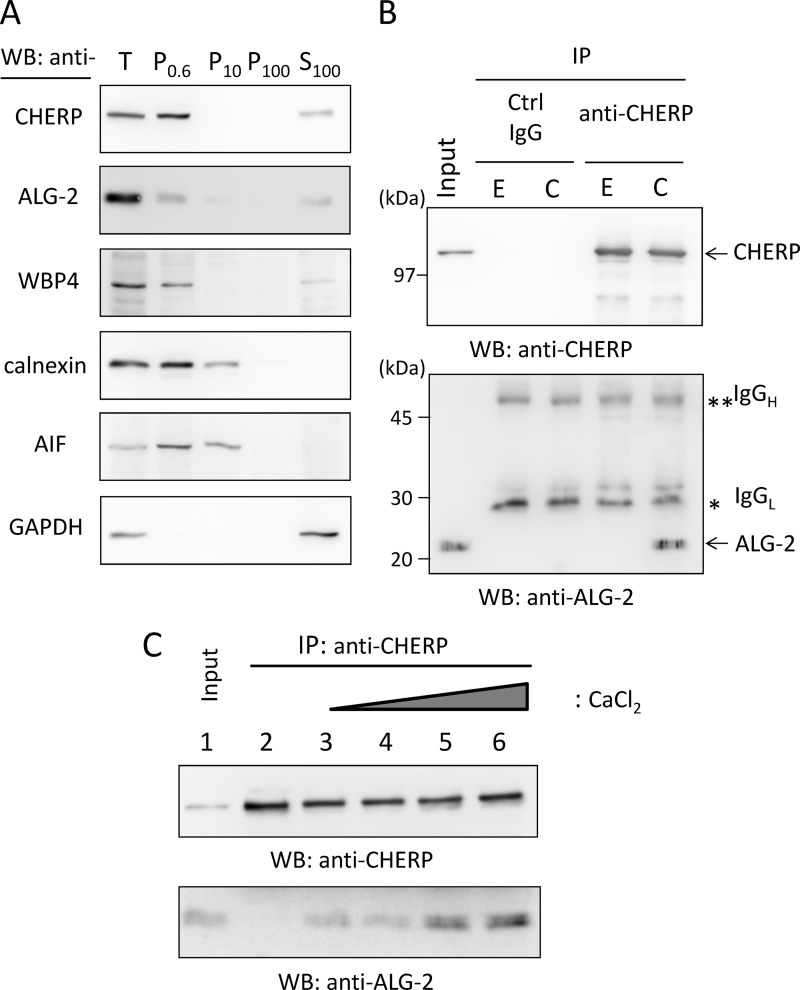
FIGURE 1.
Ca2+-dependent interaction between ALG-2 and CHERP. A, subcellular fractionation of endogenous CHERP. HEK293T cells were homogenized and fractionated by differential successive centrifugations at 4 °C. Pellets of 600 × g (P0.6), 10,000 × g (P10), 100,000 × g (P100), and the final supernatant of 100,000 × g (S100) were subjected to WB with antibodies against CHERP, ALG-2, WW domain-binding protein 4 (WBP4), calnexin, apoptosis-inducing factor (AIF), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B, co-immunoprecipitation (IP) of endogenous CHERP and ALG-2. CHERP was extracted from the P0.6 fraction by brief sonication with lysis buffer T (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1.5 mm MgCl2, and 0.2% Triton X-100 containing protease inhibitors). After centrifugation at 10,000 × g for 10 min at 4 °C, the supernatant (Input) was treated with RNase A (10 μg/ml) and supplemented with 5 mm EGTA (E) or 10 μm CaCl2 (C). Each sample was subjected to immunoprecipitation by incubating first with mouse IgG (negative control, ctrl) or anti-CHERP mAb for 1 h at 4 °C and then with magnetic beads carrying protein G overnight. After the beads had been collected and washed, immunoprecipitated proteins (IP products) were subjected to SDS-PAGE using 12.5% gel, transferred to a sheet of PVDF membrane, which was cut into halves (high and low molecular weight), and subjected to WB with anti-CHERP mAb (upper panel) and with anti-ALG-2 pAb (lower panel). Single and double asterisks indicate light and heavy chains of IgG, respectively. The relative amount of cleared cell lysate proteins (Input) used for analysis of IP products was 1.5%. C, analysis of Ca2+ dependence of binding between ALG-2 and CHERP. Nuclear extracts were supplemented with 5 mm EGTA (lane 2) or with different concentrations of CaCl2 (lane 3, none; lane 4, 1 μm; lane 5, 10 μm; lane 6, 100 μm) and subjected to co-immunoprecipitation with anti-CHERP mAb. The relative amount of cleared cell lysate proteins (Input) used for analysis of IP products was 1.5%. Representative data obtained from three (A), two (B), and four (C) independent experiments are shown.