FIGURE 5.
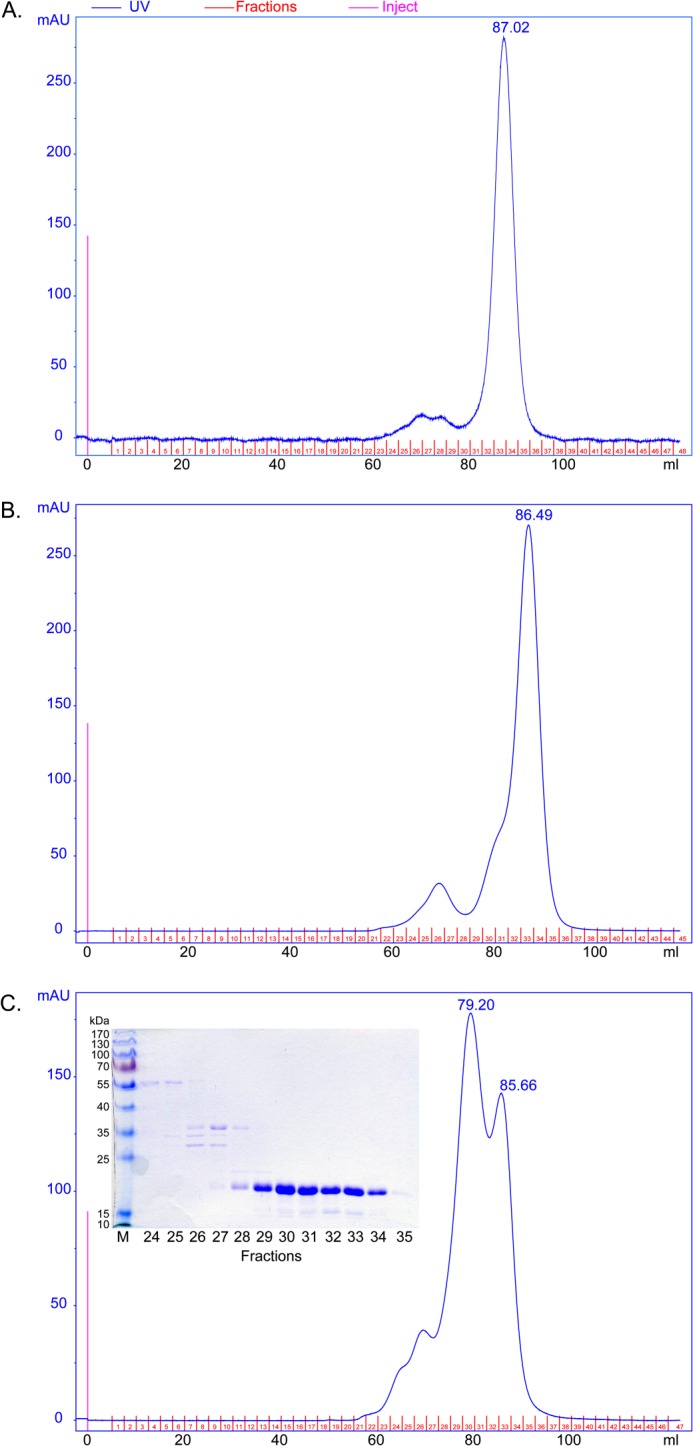
Gel filtration experiments of AgamOBP48. The concentrated pooled elution fraction from ion exchange chromatography was applied immediately (A), after 48 h (B), and after 96 h (C) of storage at 4 °C, to a calibrated Superdex 75 16/73 column. A, AgamOBP48 was eluted as a single peak corresponding to a monomer with apparent mass of 24.4 kDa. The monomer self-associates to form dimers as indicated by the appearance of a left shoulder (B) and a predominant peak (C), corresponding to a dimer with apparent mass of 40.7 kDa. Inset, SDS-PAGE analysis (12% stained with Coomassie) of the eluted fractions showed that both peaks contain AgamOBP48 protein with apparent mass of about 20 kDa. mAU, milliabsorbance units.
