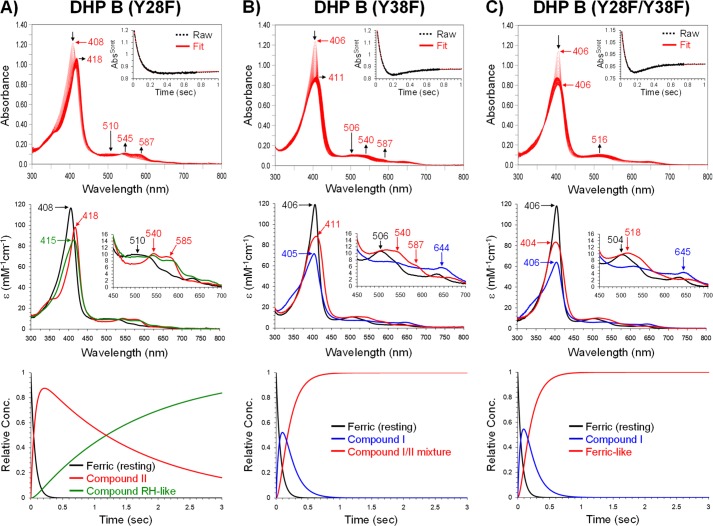
FIGURE 6.
Kinetic data obtained by optical spectroscopy for the DHP B mutants. A, DHP B (Y28F): top panel, stopped-flow UV-visible spectra of 10 μm enzyme reacting with a 10-fold excess of H2O2 at pH 7.0 (400 scans over 3 s); inset, the single wavelength (407 nm) dependence on time obtained from the raw spectra and its fit with a superposition of the calculated spectra components; middle panel, calculated spectra of the three reaction components derived from the SVD analysis: ferric heme state (black), ferryl heme or Compound II (red), and a species reminiscent of Compound RH (green); bottom panel, time dependences of the relative concentrations for the three components shown in the middle panel as determined by fitting the spectra set in the top panel. B, DHP B (Y38F): top, middle, and bottom panels are similar to those in A, whereas the three reaction components in the SVD analysis were ferric heme state (black), Compound I (blue), and a species reminiscent of Compound II (Compound I/II mixture, red). C, DHP B (Y28F/Y38F): top, middle, and bottom panels are similar to those in A. The three reaction components in the SVD analysis were ferric heme state (black), Compound I (blue), and a “Ferric-like” heme species (red).