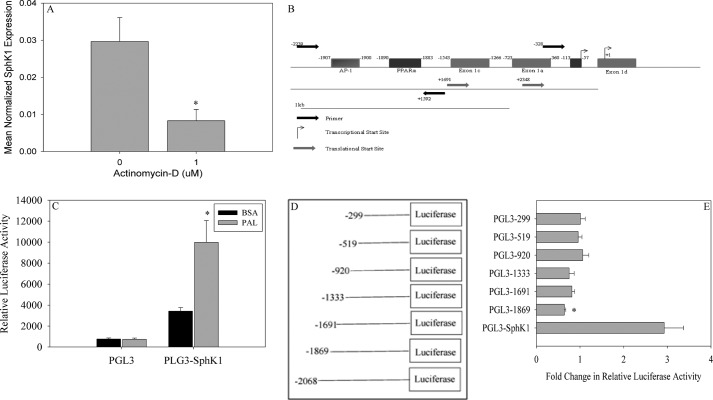
FIGURE 2.
PAL induces SphK1 through activation of the promoter. A, to evaluate the role of transcription in PAL-induced Sphk1 expression, C2C12 myoblasts were treated with 0.75 mm PAL in the presence or absence of 1 μm actinomycin D for 16 h. Following treatment, cells were harvested and the expression of Sphk1 was analyzed by qPCR. Data are presented as mean normalized expression ± S.E. (n = 3). *, p < 0.01 versus non-actinomycin D-treated cells. B, the murine Sphk1 promoter was cloned based on sequence homology with the rat Sphk1 promoter. The cloned promoter was cloned into a PGL3 basic vector for further use. The region of the promoter necessary for PAL-induced Sphk1 promoter activation was analyzed for transcription factor responsive elements using the transcription factor prediction model ALGGEN-PROMO (54, 56). C, C2C12 myoblasts were transfected with empty PGL3 or the PGL3-Sphk1 vector. Cells were treated with BSA or 0.75 mm PAL 18 h post-transfection for 8 h. Cells were assayed for relative luciferase activity and normalized to β-galactosidase activity. Data are presented as fold-change in relative normalized luciferase activity ± S.E. (n = 3). *, p < 0.05 versus PGL3-Sphk1 BSA cells. D, systematic deletions were generated of the Sphk1 promoter and cloned into the PGL3 vector. E, previously generated deletion constructs were transfected into C2C12 myoblasts. Eighteen hours post-transfection, cells were treated with BSA or 0.75 mm PAL for 8 h and assayed for relative luciferase activity. Measured luciferase activity was normalized to β-galactosidase activity. Data are presented as fold-change in normalized relative luciferase activity ± S.E., (n = 6). *, p < 0.05 versus PGL3-Sphk1.