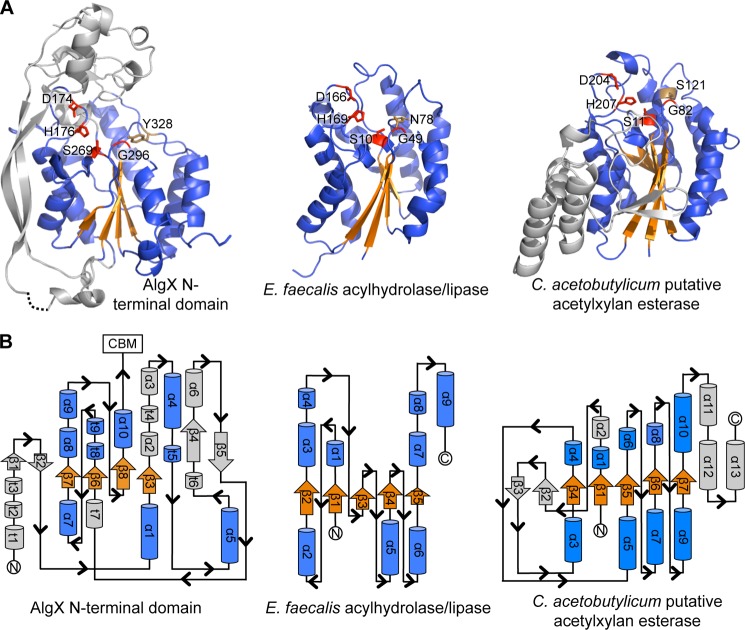
FIGURE 4.
The N-terminal domain of AlgX has an SGNH-like hydrolase fold. A, the N-terminal SGNH hydrolase-like domain of AlgX and two representative members of the SGNH hydrolase superfamily: E. faecalis acylhydrolase/lipase (PDB code 1YZF) and the putative acetylxylan esterase from Clostridium acetobutylicum (PDB code 1ZMB). In all three proteins, the Ser-His-Asp triad and signature Gly residues are structurally conserved and shown in red in stick representations. The conserved SGNH Asn residue and the equivalent Tyr in AlgX are shown in brown in stick representations. The parallel β-sheet and surrounding α-helices of the core SGNH hydrolase domains and equivalent features in AlgX are colored in orange and blue, respectively. The secondary structural elements of AlgX and the C. acetobutylicum protein that are not part of the core SGNH hydrolase fold are colored in gray. B, topology of the N-terminal domain of AlgX and its comparison with the typical topologies found in the SGNH hydrolases, E. faecalis acylhydrolase/lipase and C. acetobutylicum putative acetylxylan esterase. The topology diagrams are colored as in A.