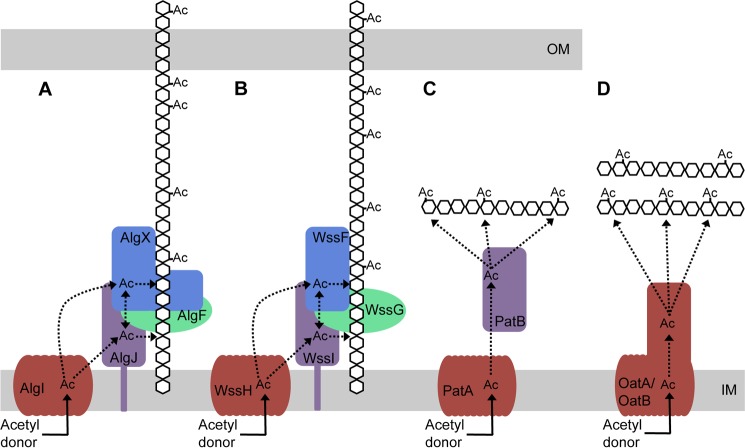
FIGURE 8.
Model for O-acetylation of alginate and other bacterial polysaccharides. A, AlgI receives an acetyl group from a cytoplasmic acetyl donor. From AlgI, the acetyl group is transferred to AlgJ and/or AlgX and then on to the alginate polymer. AlgJ and AlgX may be able to pass the acetyl group between themselves. The role of AlgF is currently unknown. B, the four proteins of the P. fluorescens Wss system acetylate cellulose and may act in a similar manner to the four Alg proteins. C, proposed mechanism of peptidoglycan acetylation in Gram-negative bacteria as presented by Moynihan and Clarke (81). This mechanism requires two proteins, an AlgI homologue, PatA, which transports the acetate across the cytoplasmic membrane to the periplasm, and an AlgJ homologue, PatB, which transfers the acetate to the sugar. D, in Gram-positive bacteria, the N-acetyl muramic acid residues of the peptidoglycan are O-acetylated by the two-domain protein, OatA (80, 94). The N-terminal domain of OatA is homologous to AlgI, and the C-terminal domain is homologous to AlgJ (80). More recently, a second protein, OatB, has been identified in Lactobacillus plantarum, which O-acetylates N-acetylglucosamine peptidoglycan residues (80). OatB also has two domains homologous to AlgI and AlgJ. Ac, acetate. Dotted lines, potential movement of the acetate after transport across the inner membrane. Proteins with similar predicted structure and function are colored the same.