FIGURE 5.
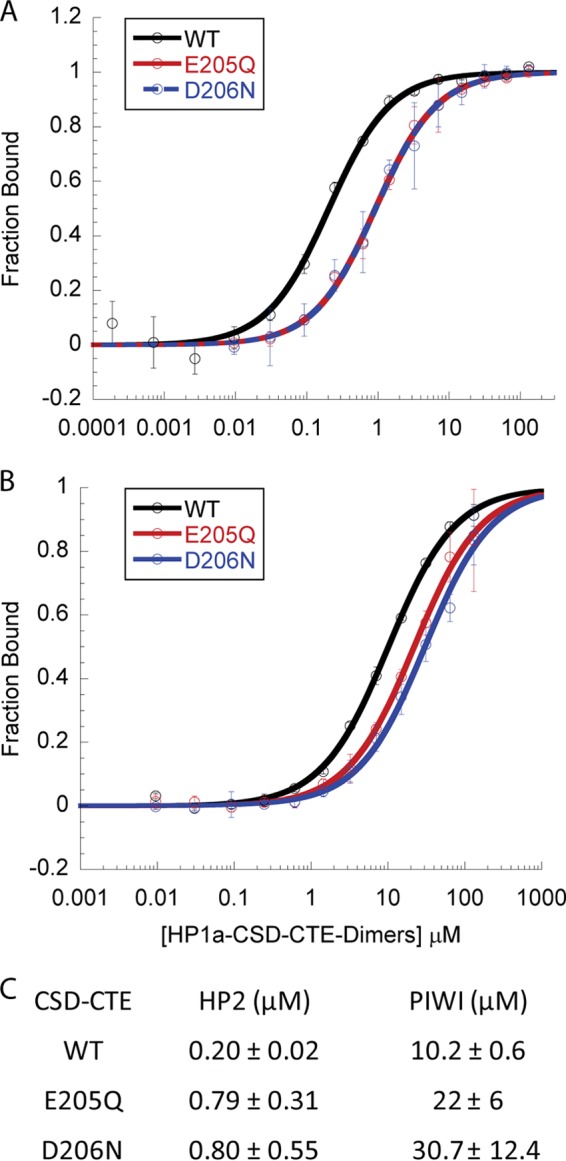
HP1a CTE charged residues enhance binding of both HP2 and PIWI. A and B, fluorescence Polarization data assessment of HP1a-CSD-CTE WT binding of HP2 (A) or PIWI (B) compared with that of CSD mutations E205Q or D206N. The fraction of peptide bound is plotted as a function of HP1a-CSD-CTE dimer. C, table of the KD values of the peptides binding to the WT, E205Q, or D206N forms of HP1a-CSD-CTE. Loss of either charged residue in the CSD results in weaker binding of both peptides.
