FIGURE 6.
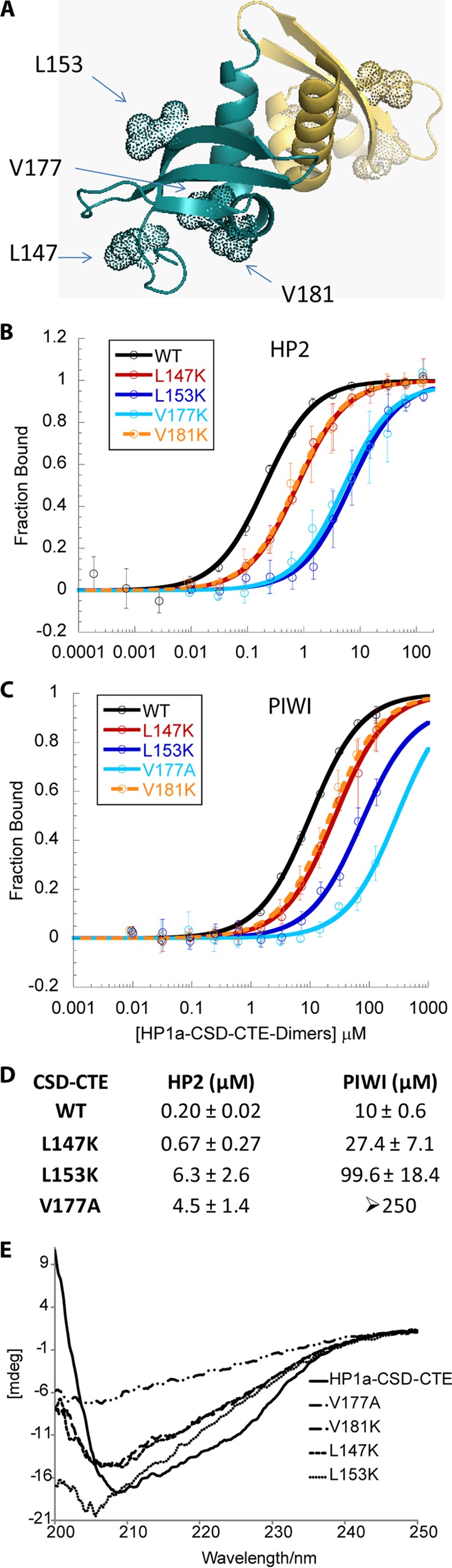
HP1a-CSD-CTE mutations L147K, L153K, V177A, and V181K all cause a decrease in binding to both HP2 and PIWI concomitant with a change in secondary structure. A, crystal structure of HP1a-CSD-CTE (PDB 3P7J). WT residues that were mutated are shown as dotted spheres. B and C, fluorescence polarization binding curves for HP2 (B) and PIWI (C) for the WT and mutant forms of the HP1a-CSD-CTE. The fraction of peptide bound is plotted as a function of HP1a-CSD-CTE dimer. D, table of the KD values of the peptides binding to HP1a-CSD-CTE WT or mutated forms. E, circular dichroism far-UV spectra of HP1a-CSD-CTE WT and mutants V147, V153, V177, and V181. These mutants exhibit a change in their CD spectrum that indicates a change in secondary structure relative to WT.
