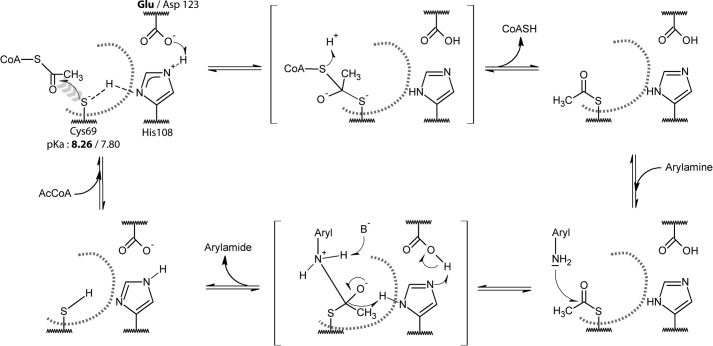
FIGURE 4.
Putative catalytic mechanism for the Glu- and Asp-containing (BACCR)NAT3 enzymes. The pKa values of the catalytic cysteine residue suggest that it exists mostly as a thiol at physiological pH (<8), which would be inconsistent with the nucleophilic attack on the AcCoA molecule (first reaction step). Thus, as suggested by Sikora et al. (43) for the M. tuberculosis NAT enzyme, we propose that the Glu/Asp123 and His108 dyad act as general base that facilitates the formation of a thiolate. In the absence of AcCoA in the active site, the thiol proton is shared between Cys60 and His108 (dashed line). The AcCoA binding in the active site could displace this equilibrium and help in the deprotonation of the thiol, leading to the nucleophilic attack of the thiolate on the AcCoA. The following catalysis steps are likely to involve the canonical tetrahedral intermediate states (in brackets) that are known to occur in the Ping Pong Bi Bi mechanism of NAT enzymes. The active site is represented by a gray dashed curve, the His108 and Glu/Asp123 residues are buried, and the Cys69 is solvent-accessible.