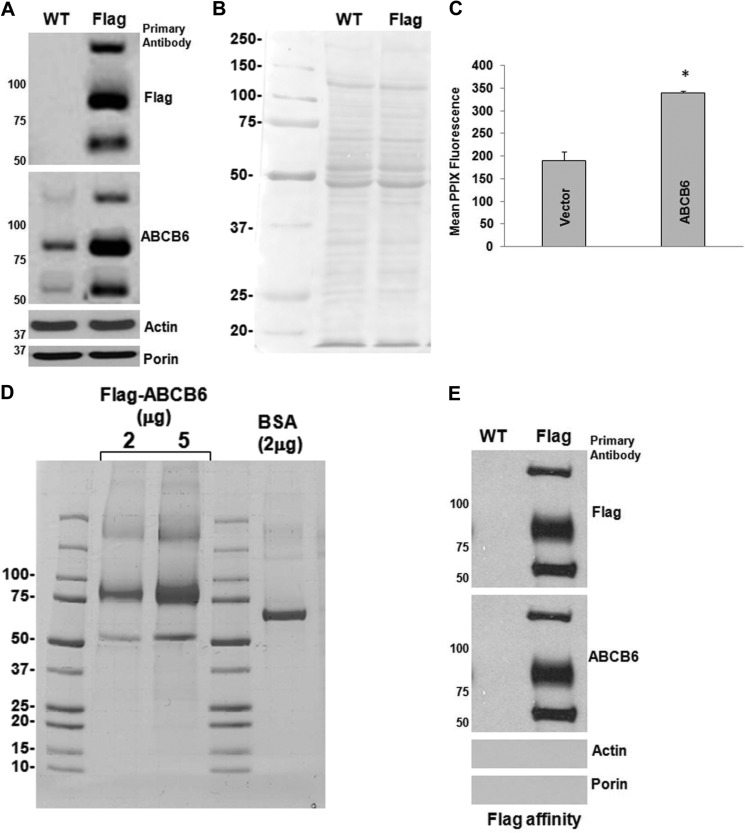
FIGURE 1.
Expression and purification of ABCB6 from total cell fraction. Total cell fractions were analyzed by SDS-PAGE (4–15%) followed by immunoblotting (A) and Ponceau staining (B). 50 μg of protein were applied per lane. Ponceau staining was used to confirm uniform loading of samples. Actin and Porin are cellular and mitochondrial proteins serving as controls. C, ABCB6-FLAG-overexpressing cells show increased heme synthesis compared with vector control cells. Values represent the mean ± S.D. *, significantly different from vector control cells; p < 0.01. D and E, solubilization and purification of FLAG-tagged ABCB6 via FLAG-affinity chromatography are shown. D, 2 and 5 μg of protein eluted from the affinity column was analyzed by SDS-PAGAE (4–15%) followed by Coomassie staining. BSA (2 μg) used as a loading control for the estimation of protein concentration is shown on the same gel. Affinity-purified ABCB6-FLAG protein bands (at 180, 90, and 50 kDa) were identified as ABCB6 by immunoblotting (500 ng-purified protein) using ABCB6-specific antibody and peptide mass fingerprinting (E). Results are representative of three independent experiments.