FIGURE 1.
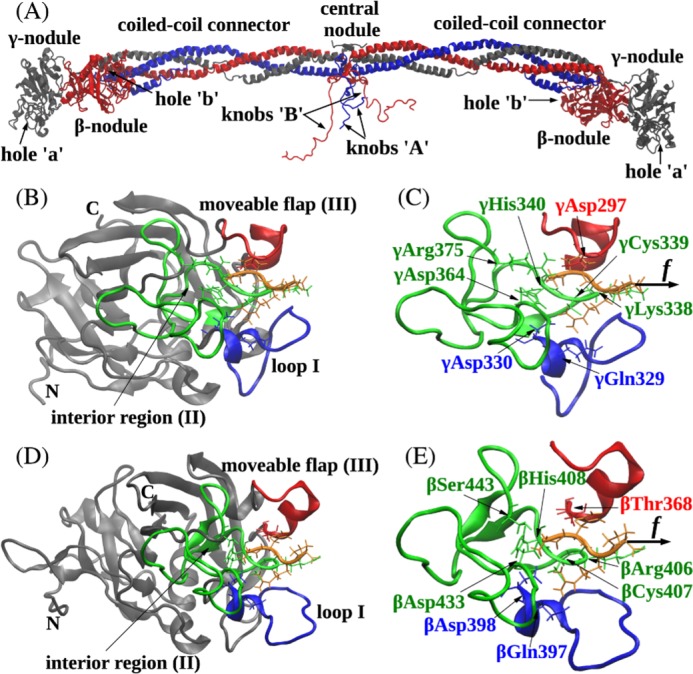
Ribbon structures of fibrin(ogen) (A), the A:a knob-hole bond (B and C), and the B:b knob-hole bond (D and E). The structures correspond to the A:a knob-hole complex (model system Aa1) and B:b knob-hole complex (system Bb1), respectively, at pH 7 and T = 25 °C. B and D, the interface of the A:a knob-hole complex (B) and B:b knob-hole complex (D) in which the binding determinants, loop I (region I shown in blue), interior region (region II shown in green), and moveable flap (region III shown in red), interact with peptides GPRP and GHRP (shown in orange), respectively. C and E, simulation setup. Holes ‘a’ and ‘b’ are constrained through fixing the C termini of the γ chain (residue γGly160) and β chain (residue βVal205), respectively (see “Experimental Procedures”). A constant pulling force f (represented by the black arrow) is applied to the Pro4 residue of GPRP peptide and Pro4 residue of GHRP peptide in the direction perpendicular to the binding interface to dissociate the knob-hole bond. Also shown are structural details of A:a and B:b knob-hole bonds in which residues in binding regions I–III in holes ‘a’ and ‘b’ establish binding contacts with peptides GPRP and GHRP.
