FIGURE 6.
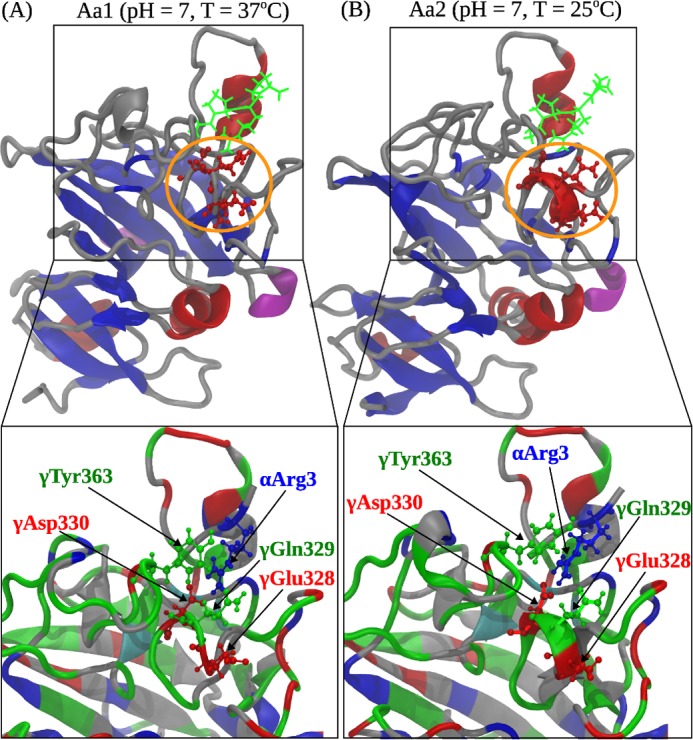
Comparison of the A:a knob-hole interactions in neutral solution (pH 7) at T = 37 °C (model system Aa1; A) and T = 25 °C (model system Aa2; B). Shown are the ribbon structures of binding site ‘a’ interacting with knob ‘A’. Color denotation is as follows: in hole ‘a’, α-helices are shown in red color, β-strands are in blue color, and coils and turns are shown in gray color; knob ‘A’ is displayed in green color. Residues γ327–330 in loop I form an α-helix at 25 °C but transition to a random coil structure at 37 °C. Interacting residues in loop I and GPRP are magnified below. The electrostatic coupling among residue γTyr363; residues γGlu328, γGln329, and γAsp330 in loop I; and residue Arg3 in the GPRP peptide is indicated.
