FIGURE 2.
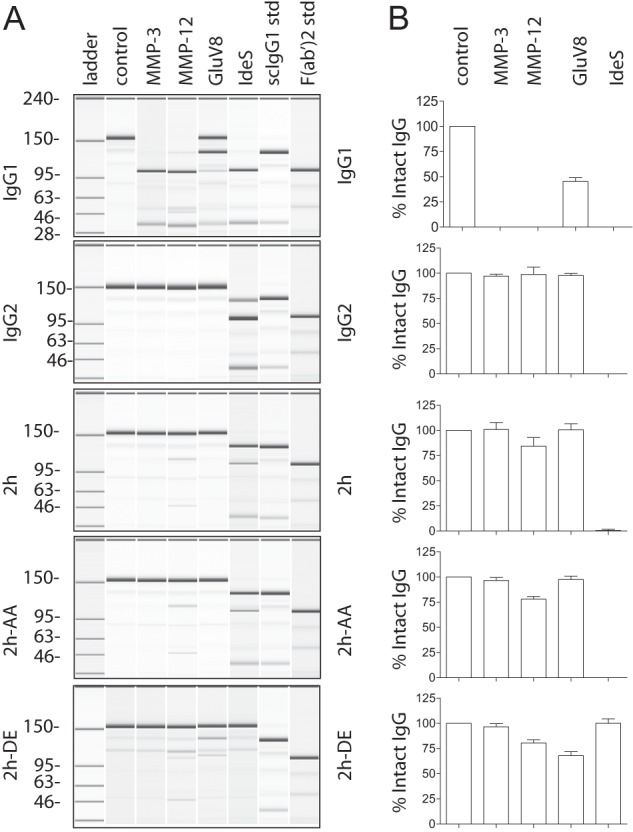
Replacement of the lower hinge/proximal CH2 amino acids of IgG1 with those of IgG2 confers protease resistance. A, purified human IgG1, IgG2, 2h, 2h-AA, and 2h-DE were incubated with different proteases and analyzed by capillary electrophoresis under denaturing, non-reducing conditions. Enzymes are listed above individual lanes, and all digestions were carried out for 24 h at 37 °C. The far right two lanes represent the purified human IgG1 standards (std) of single cleaved IgG1 and F(ab′)2 fragment of IgG1, respectively. B, bar graph representation of the percentage of intact IgG remaining after the 24 h digests (n = 3). Error bars represent S.D.
