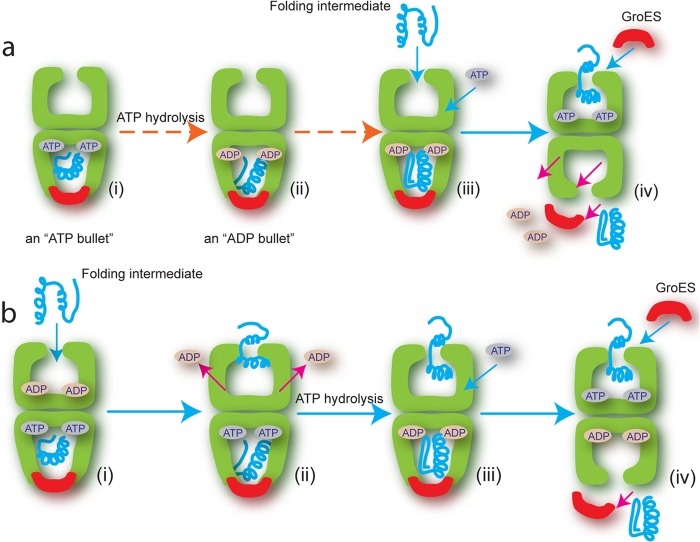
FIGURE 1.
Comparison of conventional and revised reaction sequence for protein entry into the GroEL folding reaction. a, in the conventional model, the reaction begins with the product of the previous cycle: the asymmetric GroEL (green)-ATP-GroES (red) complex (an ATP bullet) contains substrate protein (blue) within the cis cavity (i). ATP hydrolysis results in the GroEL-ADP-GroES substrate-bound complex (an ADP bullet) (ii), which now captures the next non-native substrate protein (Folding intermediate) on the open, nucleotide-free trans ring (iii) (15). ATP binding to the trans ring is required for the next round of GroES binding, substrate encapsulation and folding. Although the order in which ATP and substrate protein bind is unclear, both must bind the trans ring to trigger release of GroES and substrate protein from the cis ring (iv). b, in the revised model, the reaction begins with the asymmetric ATP bullet complex, but with ADP tightly bound to the trans ring (i) (18, 19). Binding of the non-native protein drives ADP off the trans ring (ii). ATP binds the trans ring only after ATP hydrolysis on the cis ring has generated an ADP bullet (iii), a reaction that is inhibited by ADP bound to the trans ring (29). GroES then encapsulates the substrate protein to initiate folding and simultaneously trigger release of GroES and substrate protein from the cis ring (iv).