FIGURE 5.
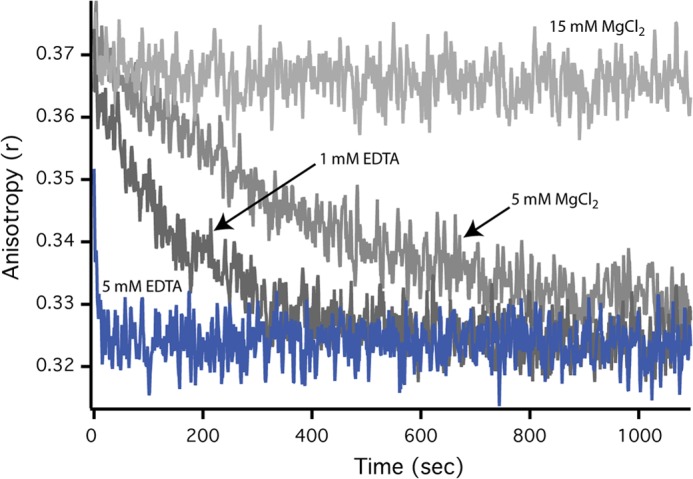
Disassembly of SR1-GroES complex and release of substrate protein is fast at 4 °C with EDTA. The dissociation rate of the SR1-GroES complex under different conditions was examined by following the rate of release of an encapsulated substrate protein. First, acid-denatured GFP (500 nm) was bound to SR1 (2.5 μm). The complex was then mixed with ATP (2 mm) and GroES (10 μm), followed by incubation of the sample at 25 °C for 5 min. SR1-GroES complexes containing folded and fluorescent GFP were purified away from free GFP using gel filtration chromatography as previously described (8). Samples of the purified SR1-GroES-GFP complex were then incubated at 4 °C in the presence or absence of EDTA and MgCl2 to determine the rate of complex disassembly and substrate protein release from an artificially disrupted SR-GroES complex. GFP release into free solution was observed as a decrease in steady state fluorescence anisotropy (15).
