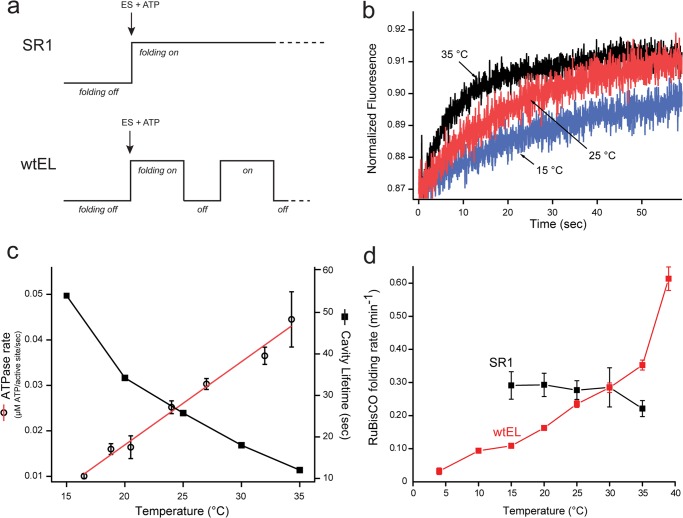
FIGURE 6.
The cycling, wild-type GroEL-GroES system folds RuBisCO at a rate that exceeds the maximum folding rate possible from confinement within the static SR1-GroES cavity. a, schematic summarizing a key difference between assisted protein folding with the single ring GroEL mutant and the wild-type GroEL system. Although a productive SR1-GroES folding cavity can be maintained for an arbitrary duration, wild-type GroEL-GroES cycles, ejecting substrate protein with each cycle (12, 13). At 25 °C, the cycle period is ∼25–30 s when substrate protein is limiting (15, 27). b, the lifetime of the GroEL-GroES complex under conditions of steady state ATP hydrolysis was measured over a range of temperatures, using a previously described FRET assay (15). In the absence of substrate protein, the extent of energy transfer between a donor-labeled GroEL variant (150 nm) and an acceptor-labeled GroES variant (150 nm) in the presence of ATP (2.5 mm) was monitored as a function of time, following addition of excess unlabeled GroES (3 μm). c, lifetime measurements (closed squares) from experiments such as those in b are plotted along with the observed steady state rate of ATP turnover (open circles) by GroEL-GroES in the absence of substrate protein, as a function of temperature. Error bars show the S.D. of n = 3 replicates. d, temperature dependence of RuBisCO folding within the SR1-GroES cavity and in the presence of a fully cycling wild-type GroEL-GroES system. Non-native RuBisCO (100 nm) was bound to either excess SR1 (400 nm) or excess wtEL (400 nm). Samples were then equilibrated at the indicated temperature, and folding was initiated by the addition of a 2-fold molar excess of GroES (800 nm), plus ATP (1 mm). The extent of RuBisCO folding was sampled over the course of 30 min at the indicated temperature. In all cases, the observed refolding kinetics were well described by a single exponential rate law. The rate constant for RuBisCO folding, with either wild-type GroEL or SR1, is displayed. Error bars show the S.D. for n = 3–5 replicates. The SR1 reaction could not be reliably examined over the full temperature range, because of thermally induced disassembly of the SR1-GroES complex (based on gel filtration; data not show), which results in artificially low refolding rates.