Background: Lu/B-CAM is a specific receptor for laminin α5, a subunit of laminin-511 (LM-511) that is a major component of basement membranes.
Results: Expression of Lu/B-CAM promotes tumor cell migration on LM-511 rather than cell attachment.
Conclusion: Tumor cell migration on LM-511 requires that Lu/B-CAM competitively weakens cell attachment through integrins.
Significance: The competitive interaction of the laminin receptors may provide a balance between static and migratory cell behaviors.
Keywords: Cell Adhesion, Cell Migration, Extracellular Matrix, Integrin, Invasion, Laminin
Abstract
Cell-matrix interactions are critical for tumor cell migration. Lutheran (Lu), also known as basal cell adhesion molecule (B-CAM), competes with integrins for binding to laminin α5, a subunit of LM-511, a major component of basement membranes. Here we show that the preferential binding of Lu/B-CAM to laminin α5 promotes tumor cell migration. The attachment of Lu/B-CAM transfectants to LM-511 was slightly weaker than that of control cells, and this was because Lu/B-CAM disturbed integrin binding to laminin α5. Lu/B-CAM induced a spindle cell shape with pseudopods and promoted cell migration on LM-511. In addition, blocking with an anti-Lu/B-CAM antibody led to a flat cell shape and inhibited migration on LM-511, similar to the effects of an activating integrin β1 antibody. We conclude that tumor cell migration on LM-511 requires that Lu/B-CAM competitively modulates cell attachment through integrins. We suggest that this competitive interaction is involved in a balance between static and migratory cell behaviors.
Introduction
The invasion of tumor cells through the basement membrane is a critical step in many forms of metastasis. To invade, tumor cells require different functions, such as matrix degradation, cell migration, and cell adhesion. So far, the reconstituted basement membrane Matrigel has been used to investigate tumor invasion in vitro (1). Matrigel, an extract derived from mouse Engelbreth-Holm-Swarm sarcoma, is composed of type IV collagen, laminin, nidogen, and perlecan, which are the major components of the basement membrane. Of these components, laminin has been thought to be a key molecule mediating cell adhesion and cell migration during tumor invasion. Laminins are a family of heterotrimeric glycoproteins composed of α, β, and γ chains. There are five α chains, three β chains, and three γ chains known at present (2). To date, 19 different laminin heterotrimers have been identified in various cultured cells and tissues (3). The laminin heterotrimer in Matrigel is composed of α1, β1, and γ1 chains (laminin-111, LM-111) and is mainly expressed in fetal but not adult tissues. Hence, despite a wealth of accumulated studies, tumor cells only rarely interact with LM-111 in the process of tumor invasion. In contrast, laminin-511 (α5, β1, γ1; LM-511) has been found to be a major isoform in many adult basement membranes (4, 5). However, the nature of the interactions between tumor cells and LM-511 in invasion processes is still unclear.
Many of the biological functions of LM-511 are mediated through the α5 subunit. Mice lacking laminin α5 die during late embryogenesis with several developmental defects, including defects in neural tube closure, digit separation, placentation, glomerulogenesis, lung lobe separation, intestinal smooth muscle development, tooth morphogenesis, salivary gland morphogenesis, and bile duct maturation (6, 7). Experiments that bypass embryonic lethality have shown that laminin α5 is required for hair follicle development and lung maturation. Moreover, a hypomorphic Lama5 mutation causes polycystic kidney disease (8). These results suggest that laminin α5 plays multiple functional roles in development and establishment of tissue architecture. In addition, many studies have shown that the expression of laminin α5 is often maintained or even increased in advanced tumors (9). We also showed that laminin α5 was ectopically deposited in well and poorly differentiated hepatocellular carcinomas (10). However, the role of laminin α5 in tumor progression is unclear.
The studies of developing organs in laminin α5-deficient mice have shown that laminin α5 modulates the Sonic hedgehog pathway, the Wnt pathway, and the PI3K/Akt pathway (11, 12). In vitro studies have shown that LM-511 triggers the phosphorylation of p130cas, leading to the activation of Rac1 and PI3K/Akt, which are involved in cell migration and survival (13, 14). The interaction of cells with LM-511 is mediated by various receptors, including integrin α3β1, α6β1, and α6β4 (15, 16); α-dystroglycan (17); and Lutheran/basal cell adhesion molecule (Lu/B-CAM)2 (18–20). Lu/B-CAM is an Ig superfamily transmembrane protein in which the extracellular domain contains one variable, one constant-1, and three intermediate Ig-like domains, V-C1-I-I-I (21–23). A splice variant of Lu known as B-CAM (24) has the same extracellular and transmembrane domains as Lu, but it lacks the COOH-terminal 40 amino acids of the cytoplasmic tail. Lu has been studied mainly as the antigen of the Lutheran blood group system and in the context of sickle cell disease. On the other hand, B-CAM was identified as an up-regulated antigen in ovarian carcinoma, suggesting its involvement in tumor progression (24). However, although the interaction between laminin α5 and Lu/B-CAM is expected to be involved in tumor invasion and metastasis, it is still unproven.
Here we established a human fibrosarcoma cell line with a Flp recombination site integrated into the genome and generated stable cell lines expressing Lu or B-CAM using Flp recombinase. The cell lines allowed us to examine the functions of Lu/B-CAM in tumor cells adhering to LM-511. Although Lu/B-CAM slightly suppressed cell adhesion to LM-511, both molecules promoted cell migration with pseudopods. We also examined whether the expression of Lu/B-CAM in tumor cells affected cell migration on LM-511 using function-blocking antibodies. We found that competition between Lu/B-CAM and integrins for binding to laminin α5 modulated cell migration. We provide a possible mechanism that explains in part how tumor cells invade into bona fide basement membranes containing laminin α5.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Monoclonal antibodies against Lu/B-CAM (clones 87207 and BRIC221) were purchased from R&D Systems (Minneapolis, MN) and Serotec (Oxford, UK), respectively. Polyclonal antisera against the intercellular or extracellular domain of Lu/B-CAM have been described (10, 25). IgG purified from the antiserum was labeled with a biotinylation kit (GE Healthcare, Piscataway, NJ). Monoclonal antibodies to human integrin α3 (clone P1B5), β1 (clone 6S6), and β4 (clone ASC-3) and a polyclonal antiserum to integrin α3 were purchased from Millipore (Temecula, CA). Rat monoclonal antibodies to human integrin α6 (clone GoH3) and β1 (clone mAb13) were from BD Biosciences. Anti-human integrin β1 (clone TS2/16) was purified on a Protein-G-Sepharose 4B column from the conditioned medium of hybridoma cells purchased from the ATCC. Recombinant LM-511 was produced in HEK293 cells triple-transfected with mouse laminin α5, β1, and γ1 chains and purified as described previously (26). Human laminin-3A32 (LM-332) was purchased from Oriental Yeast (Tokyo, Japan). Human fibronectin was purchased from BD Biosciences. Trypsin (sequencing grade) was purchased from Roche (Mannheim, Germany). Recombinant proteins containing the Lu/B-CAM extracellular domain fused with a 6× His tag (Sol-Lu) or a Fc-Tag (Lu-Fc) were produced and characterized as described previously (20, 26). Cell dissociation buffer (CDB), which is protease-free Hanks' balanced salt solution containing EDTA, was purchased from Invitrogen.
Trypsin or CDB Treatment of Sol-Lu and Binding Assays on Tissue Sections
Following the protocol of the manufacturer, 1.0 μg of Sol-Lu was mixed with 0.5 or 5 ng of trypsin in 20 μl of 100 mm Tris-HCl (pH = 8.5) buffer. 1.0 μg of Sol-Lu was also mixed with half-diluted CDB. The mixtures were incubated at 37 °C for 1 h. The trypsin reaction was stopped by addition of aprotinin. Sol-Lu treated with trypsin or CDB was used for SDS-PAGE and binding assays. Mouse kidney sections were prepared as described in our previous study (27). Animal studies were permitted by the Animal Research Committee of Tokyo University of Pharmacy and Life Sciences. The sections were incubated with a mixture of Lu-Fc and the trypsin- or CDB-treated Sol-Lu. Lu-Fc bound to endogenous laminin α5 was detected with anti-human IgG antibody conjugated with Alexa Fluor 488. Quantitation of the recombinant proteins bound to laminin α5 was performed as described in our previous study (27).
Cell Culture
A549 and HT1080 cells were purchased from the Health Science Research Resources Bank (Osaka, Japan). Both cell lines were maintained in DMEM containing 10% FBS and gentamycin (Invitrogen). For subculturing, the cells were removed with CDB and plated in culture dishes.
Construction of Expression Vectors
The DNA fragment encoding Lu or B-CAM was amplified with 5′-GGGGTACCGCCACCATGGAGCCCCCGGACGCACCG-3′ (sense) and 5′-CGGGATCCTCAGCACTCGTCTCCGAAGCCCCC-3′ (antisense) or 5′-CGGGATCCTCACGGAGCCCCCTTCTCCCGC-3′ and digested with KpnI and BamHI. Each DNA fragment was inserted into the KpnI and BamHI sites of pcDNA5/Flp recombination target for a stable high-expression Flp-In system (Invitrogen). For PCR, KOD plus DNA polymerase (TOYOBO, Osaka, Japan) was used according to the instructions of the manufacturer.
Generation of Stable Expression Cell Lines
The Flp-In system (Invitrogen) was used for the generation of a stable Lu or B-CAM expression cell line. To generate a HT1080 cell line containing an integrated Flp recombination target site, the cells were transfected with the pFRT/lacZeo vector using Lipofectamine 2000 (Invitrogen), and a stable clone was selected using 100 μg/ml zeocin (Invitrogen). A selected clone, HT1080F, was cotransfected with the expression vector and the FLP-recombinase vector (pOG44), resulting in a stable integration of the gene of interest at the FLP recombination target site in the genome (38). Isogenic stable cell lines expressing Lu or B-CAM were selected using 100 μg/ml hygromycin (Invitrogen). Hygromycin-resistant and zeocin-sensitive cells were further assayed for lack of β-galactosidase activity.
Flow Cytometry
The cells were removed with CDB and suspended in PBS(-) containing 0.1% BSA and 1 mm EDTA. The suspended cells were incubated with antibodies for 1 h at 4 °C. After washing with PBS(-) containing 0.1% BSA and 1 mm EDTA, the cells were incubated with Alexa Fluor 488-labeled secondary antibody for 1 h at 4 °C. The cells were then analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Quantification of Cell Morphology with Pseudopods
The ratio of square area surrounding the cell body without or with pseudopods reflects cell morphology with pseudopods. To obtain the area of cells, we drew a square surrounding the cell body without (a) or with (b) pseudopods. The square area was calculated using ImageJ. The ratio of b/a was dotted, and statistical significance was determined using KaleidaGraph.
Cell Migration Assay
The cells were removed with CDB and suspended in serum-free DMEM. The cells were plated on 35-mm dishes (Nunc, Roskilde, Denmark) coated with 0.8 nm LM-511, 0.8 nm LM-332, or 40 nm fibronectin and blocked with 1% BSA. Two hours post-plating, cell migration was monitored using Biozero (Keyence, Osaka, Japan). Video images were collected with a charge-coupled device camera at 4- or 10-min intervals using BZ-Viewer and BZ-Analyzer (Keyence, Osaka, Japan). The positions of nuclei were tracked to quantify cell motility. Velocities were calculated in micrometers per 4 or 8 h using ImageJ.
Cell Adhesion and Inhibition Assays
Cell adhesion assays using LM-511, LM-332, and fibronectin were performed as described previously (26). Briefly, 96-well plates (Nunc) were incubated with proteins at 4 °C overnight and then blocked with PBS(-) containing 1% BSA for 1 h at 37 °C. The cells were removed with CDB, plated on the coated dishes, and incubated at 37 °C for 1 h. Adherent cells were fixed with 4% formaldehyde and stained with Diff-Quik (International Regents Corp., Kobe, Japan). The stained cells were counted under a microscope. For inhibition assays, 96-well microtiter plates were incubated with 0.8 nm of LM-511 or LM-332 at 37 °C for 1 h and then blocked with PBS(-) containing 1% BSA for another hour. The cells were suspended in serum-free DMEM at a density of 4 × 105 cells/ml and preincubated with 1 μg/ml of antibody at room temperature for 10 min. 50 μl of cell suspension was added to wells coated with the recombinant proteins. After incubation at 37 °C for 30 min, the attached cells were stained as described above.
Deglycosylation and Immunoblotting
For preparation of cell lysates, cells were removed from culture dishes with CDB and lysed with lysis buffer (10 mm Tris-HCl (pH 7.5), 10 mm EDTA, 150 mm NaCl, and 1% Nonidet P-40) containing a protease inhibitor mixture (Sigma). Lysates were clarified at 10,000 rpm and precleared with protein G-Sepharose (GE Healthcare). After preclearing, protein G-Sepharose and anti-Lu/B-CAM monoclonal antibody (clone BRIC221) were added to the samples. After incubation for 1 h at 4 °C, immune complexes were washed twice with the lysis buffer and dissolved with SDS-PAGE sample buffer. The immune complexes were separated on SDS-PAGE under reducing condition and transferred to a PVDF membrane. Proteins on the membrane were probed with a biotin-labeled polyclonal antibody to the extracellular domain of Lu/B-CAM. Bound antibodies were visualized with streptavidin-horseradish peroxidase (GE Healthcare).
For deglycosylation of Lu/B-CAM, the immune complexes were suspended in deglycosylation buffer (100 mm Tris-HCl (pH 8.6), 0.1% SDS, 1% Nonidet P-40, and 200 mm 2-mercaptoethanol) with 40 milliunits/ml glycopeptidase F. After incubation for 20 h at 37 °C, the deglycosylated proteins were used for immunoblotting.
Generation of Lu/B-CAM Knockout Mice and Immunohistochemistry
Production of Lu/B-CAM-null mice was described in our previous study (28). For immunohistochemistry, epidermal tissues of embryonic day 17.5 embryos were frozen in optimum cutting temperature compound. Sections were cut at 7 μm in a cryostat and air-dried. For staining, the sections were blocked in 10% normal goat serum and then incubated with the anti-cytoplasmic tail of Lu or integrin α3 polyclonal antisera. Secondary antibodies were conjugated to Alexa Fluor 488 (Invitrogen). After several washes, sections were mounted in 90% glycerol containing 0.1× PBS and 1 mg/ml p-phenylenediamine. Images were captured using a Spot 2 charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI) and imported into Adobe Photoshop Elements 10 Editor for processing.
RESULTS
The Impact of Trypsin or CDB Treatment on the Binding of Lu/B-CAM to Laminin α5
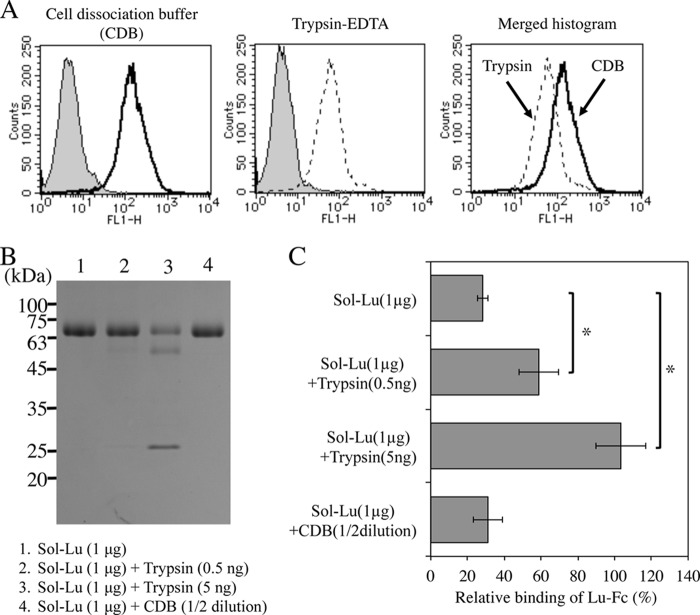
Richard et al. (29) showed that the immunoreactivity of Lu/B-CAM is reduced by trypsin, α-chemotrypsin, and ficain. Cultured cells are usually removed from dishes using trypsin. We therefore examined whether trypsin treatment influenced the binding of Lu/B-CAM to laminin α5. Consistent with the previous report, flow cytometric analysis showed that trypsinization decreased the immunoreactivity of Lu/B-CAM at the cell surface (Fig. 1A). Treatment of recombinant Sol-Lu with trypsin generated two fragments of 56 and 24 kDa, whereas Sol-Lu was not damaged in CDB (Fig. 1B). As shown in Fig. 1C, trypsin-treated Sol-Lu did not disturb the binding of Lu-Fc to laminin α5, indicating that trypsinization influenced Lu/B-CAM binding activity. This result suggests that a trypsin cleavage site is localized in the hinge region of Lu/B-CAM that is necessary for binding to laminin α5. Therefore, a CDB without trypsin was used for removing cells from culture dishes.
FIGURE 1.
The binding of trypsin-treated Lu/B-CAM to laminin α5. A, flow cytometric analyses of Lu/B-CAM expression on A549 cells prepared with either CDB or trypsin-EDTA. The expression of Lu/B-CAM is shown as a solid line (CDB) and a dotted line (trypsin-EDTA). The gray area indicates the negative control. B, SDS-PAGE of trypsin- or CDB- treated Sol-Lu. Sol-Lu was incubated with trypsin or CDB at 37 °C for 1 h, separated on a 12.5% gel under non-reducing conditions, and stained with Coomassie Brilliant Blue. Molecular weight standards are indicated. C, the binding of Lu-Fc to laminin α5 in the presence of Sol-Lu or trypsin- or CDB-treated Sol-Lu. Binding assays on tissue sections were performed, and Lu-Fc bound to laminin α5 was quantified as described under “Experimental Procedures.” The binding of Lu-Fc was set to 100 in the absence of Sol-Lu or trypsin- or CDB-treated Sol-Lu. Each bar represents the mean of triplicate assays. Error bars indicate S.D. *, p < 0.05 by Student's t test.
Overexpression of Lu/B-CAM in Human Fibrosarcoma
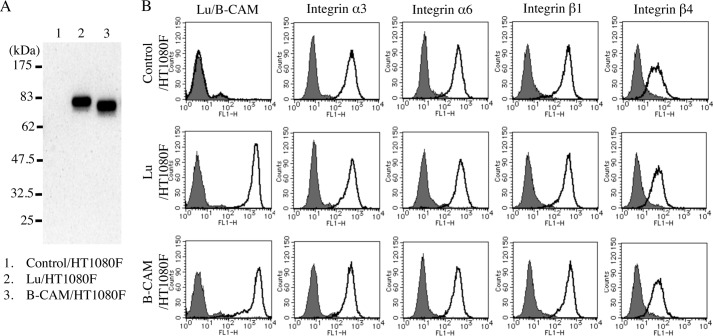
To characterize the functions of Lu/B-CAM in cell adhesion to LM-511, each gene was transfected into human fibrosarcoma HT1080 cells, which do not express either molecule. To exclude clonal variation, Lu or B-CAM was stably expressed in HT1080F cells using the Flp recombination system. A combination of immunoprecipitation and immunoblotting indicated that Lu and B-CAM migrated at the predicted relative molecular masses (Fig. 2A). Flow cytometric analysis also showed that both were expressed at comparable levels on the cell surface (Fig. 2B). Although overexpression of CD146, which shares a similar structure with Lu/B-CAM, down-regulates cell surface expression of integrins (30), Lu/B-CAM expression did not influence the levels of integrin α3, α6, β1, and β4, the major receptors for laminin α5 (Fig. 2B).
FIGURE 2.
Analysis of expression of Lu and B-CAM in HT1080F control cells and transfectants. A, immunoblot analysis of Lu/B-CAM in control/HT1080F (lane 1), Lu/HT1080F (lane 2), and B-CAM/HT1080F (lane 3) cells. Cell lysates were immunoprecipitated with anti Lu/B-CAM mAb (BRIC221). Immunoprecipitates were separated on a 10% gel under reducing conditions and immunoblotted with anti-Lu/B-CAM polyclonal antiserum. Lu and B-CAM migrated at 80 kDa and 76 kDa, respectively. Molecular weight standards are indicated. B, flow cytometric analyses of Lu/B-CAM and integrin α3/α6/β1/β4 expression. The cells were incubated with the indicated primary antibodies and then with Alexa Fluor 488-conjugated secondary antibody for flow cytometric analysis. The expression of Lu/B-CAM and integrin α3/α6/β1/β4 is shown as a solid line. The gray area indicates the negative control. Overexpression of Lu or B-CAM did not influence the expression of laminin α5-binding integrins α3β1, α6β1, or α6β4.
The Adhesion of Transfectants Expressing Lu/B-CAM to LM-511
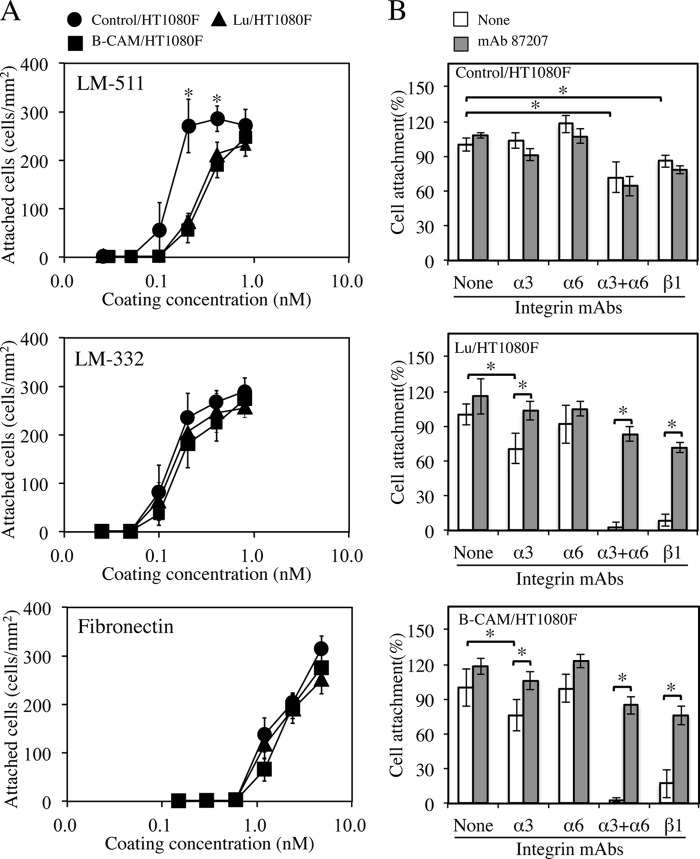
To investigate the roles of Lu and B-CAM, cell adhesion assays were performed using both transfectants. The cells were plated on dishes coated with LM-511, LM-332, or fibronectin. As shown in Fig. 3A, although the ability of cells to attach to LM-332 and fibronectin was similar between control and transfected cells, the transfected cells attached to LM-511 less than control cells did. These results suggest that the binding of Lu/B-CAM to LM-511, its only known ligand, suppresses cell adhesion to it.
FIGURE 3.
Analysis of cell adhesion. A, adhesion of cells expressing Lu or B-CAM to LM-511 (top panel), LM-332 (center panel), and fibronectin (bottom panel). 96-well plates were coated with increasing concentrations of the proteins and incubated with control/HT1080F (●), Lu/HT1080F (▴) or B-CAM/HT1080F (■) at 37 °C for 1 h. Adherent cells were stained with Diff-Quik and counted. The control/HT1080F cells attached to LM-511 more readily than the Lu/HT1080F and B-CAM/HT1080F cells. Each point represents the mean of triplicate assays. Bars show S.D. *, p < 0.05 by Student's t test. B, inhibitory effects of mAb 87207 on adhesion of transfectants to LM-511. Cells preincubated with mAb 87207 and function-blocking antibodies against the indicated integrin subunits were added to LM-511-coated wells. After incubation for 30 min, the attached cells were stained and counted. Values are expressed as percentages of the number of cells adhering in the absence of antibody. Each column represents the mean of triplicate assays. Bars show S.D. *, p < 0.05 by Student's t test. mAb 87207 restored the adhesion of Lu/HT1080F and B-CAM/HT1080F cells to LM-511 in the presence of anti-integrin mAbs.
To investigate the mechanism of adhesion of the transfectants to LM-511, we assayed the binding of receptors using function-blocking antibodies to Lu/B-CAM and to the relevant integrins. We made use of a recently discovered anti-Lu/B-CAM mAb (mAb 87207) that can inhibit the binding of Lu/B-CAM to laminin α5 (27). As we showed previously (16), the adhesion of control cells to LM-511 was significantly (but not totally) inhibited by the combination of anti-integrin α3 and α6 monoclonal antibodies or anti-integrin β1 monoclonal antibody (Fig. 3B, top panel). On the other hand, the adhesion of the transfectants to LM-511 was completely inhibited by the combination of anti-integrin α3 and α6 mAbs and by the anti-integrin β1 mAb (Fig. 3B, center and bottom panels). Interestingly, the inhibitory effects of these antibodies were significantly reduced in the presence of the mAb 87207. These results show that, although Lu/B-CAM can bind to laminin α5, the binding affinity is not sufficient for robust cell adhesion. Moreover, the binding of Lu/B-CAM to laminin α5 seems to disturb the robust cell adhesion normally mediated by integrins. This is consistent with our previous study showing that Lu/B-CAM binds to laminin α5 competitively with integrin α3β1/α6β1 (26).
The Morphology of Transfectants Expressing Lu/B-CAM on LM-511
Control cells and transfectants were spread on a LM-511-coated surface (Fig. 4A). Although the control cells displayed a well spread morphology with a greater cell-substratum contact area, the transfectants assumed a spindle-shaped morphology with several pseudopods. The reduced adhesion of the transfectants to LM-511 seemed to be due to their spindle-shaped morphology. In the presence of mAb 87207, the morphology of transfectants was similar to that of control cells. To quantify the cell morphology, we measured the ratio (b/a) of the square area surrounding the cell body without (a) or with (b) pseudopods (Fig. 4B). As shown in Fig. 4C, although many transfectants with pseudopods were observed on LM-511, not all cells had them. Importantly, the pseudopods of transfectants disappeared in the presence of mAb 87207. These results show that Lu/B-CAM promotes elongation of pseudopods on LM-511, an activity inhibited by mAb 87207.
FIGURE 4.
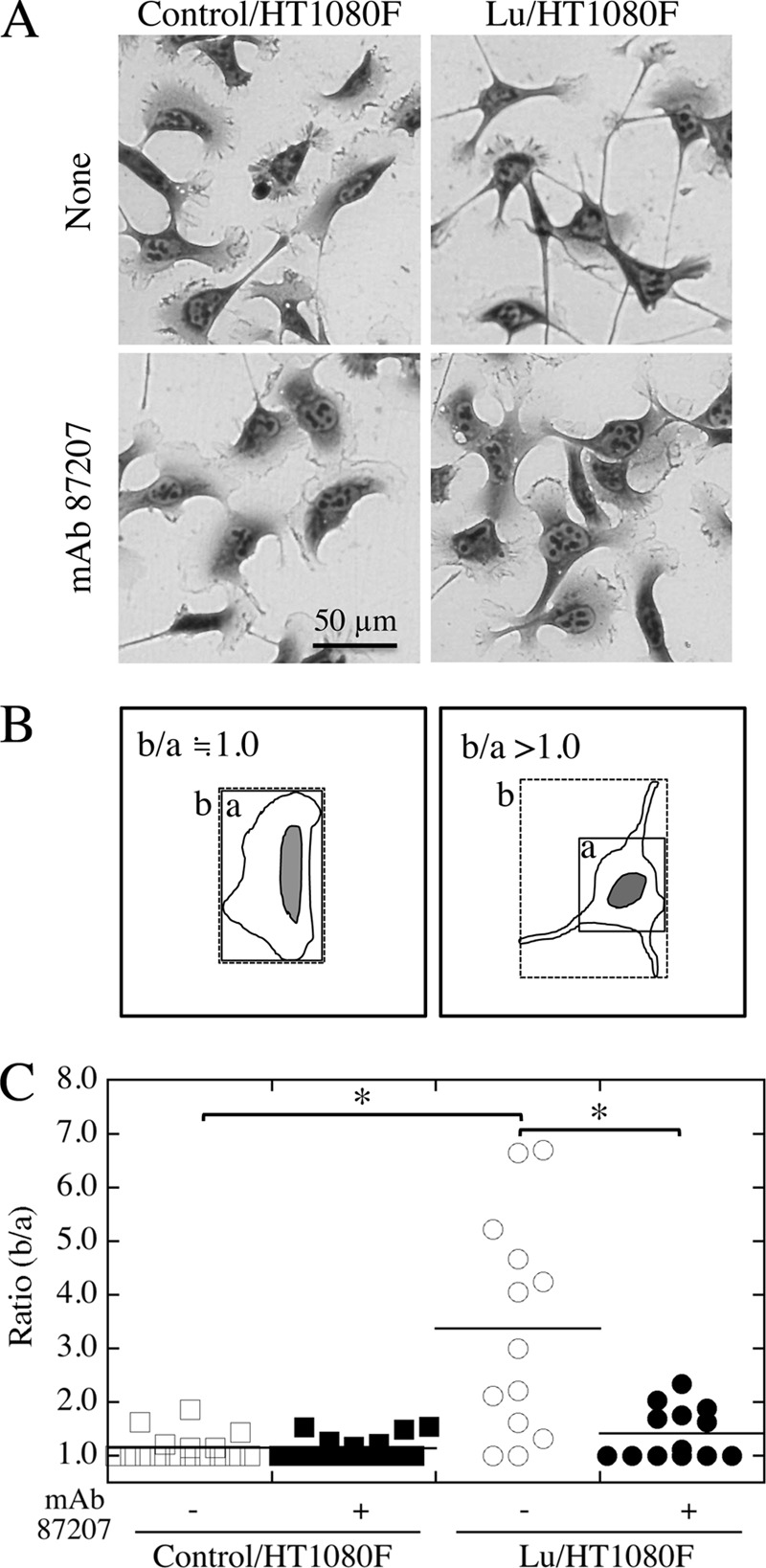
Extension of pseudopods by the transfectants on LM-511. A, morphology of cells on LM-511. The control/HT1080F (left panels) and Lu/HT1080F (right panels) cells were plated on dishes coated with LM-511 (0.8 nm). After culturing for 1 h, the cells were incubated in the absence (top panels) or presence (bottom panels) of mAb 87207. The cells were stained with Diff-Quik after another hour, and the images of cells were captured by charge-coupled device camera. The expression of Lu promoted elongation of pseudopods by HT1080F cells, and this was partly inhibited by mAb 87207. B, schematic to measure cell area ratio. The cell area with (b) or without (a) pseudopods was measured as drawn. The ratio of b/a reflects the extent of cell area with pseudopods. C, quantification of cell morphology. The images of cells in 0.05 mm2 were captured and used to measure cell area. Bars indicate means. *, p < 0.05 by Dunnett's multiple comparison test. mAb 87207 inhibited pseudopod elongation for Lu/HT1080F cells adhering to LM-511.
The Involvement of Lu/B-CAM in Cell Migration Promoted by LM-511
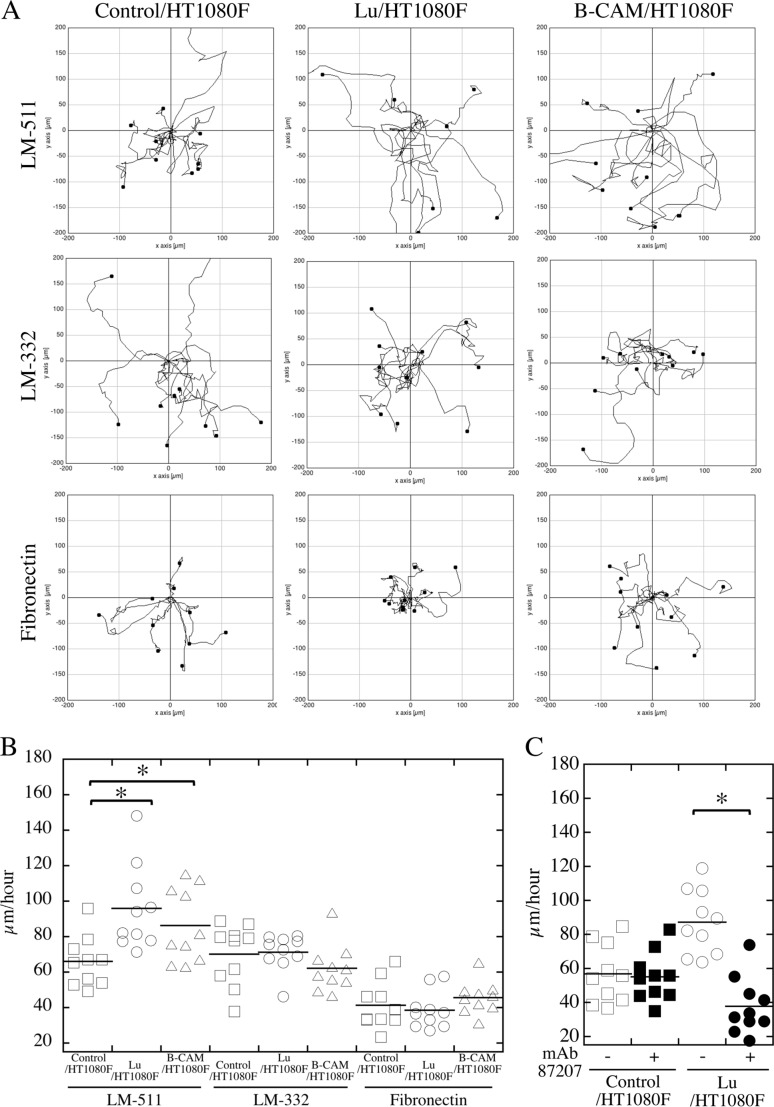
We compared the migration of the transfectants with that of the control cells using time-lapse video microscopy. The tracks of cells on LM-511, LM-332, or fibronectin were traced for 4 h (Fig. 5A). Cell velocities were calculated from the distances of tracks. The transfectants migrated faster on LM-511 than the control cells, but there was no difference on LM-332 and fibronectin (Fig. 5B). We also examined the effects of mAb 87207 on the migration of transfectants. The antibody significantly inhibited the migration of transfectants on LM-511 but showed no effect on control cells (Fig. 5C and supplemental movies 1 and 2 and their legends), indicating that the binding of Lu/B-CAM to laminin α5 promotes cell migration.
FIGURE 5.
Pattern and velocity of cell migration. The indicated cells were plated on dishes coated with LM-511 (0.8 nm, top panels), LM-332 (0.8 nm, center panels), or fibronectin (40 nm, bottom panels). Cell movements were monitored by time-lapse video microscopy. A, representative paths of cell movements on each proteins tracked at 10-min intervals over a span of 4 h. B, quantification of cell motility as evaluated by velocity (micrometers/hour) and determined using ImageJ software as described under “Experimental procedures.” *, p < 0.01 by Dunnett's multiple comparison test. C, mAb 87207 inhibited migration of Lu/HT1080F cells on LM-511. After cell adhesion to substratum, mAb 87207 was added to culture medium. After another hour, cell movements were monitored for 4 h. Cell motility was evaluated by velocity as described above.
The Involvement of Lu/B-CAM in Tumor Cell Adhesion and Migration onto LM-511
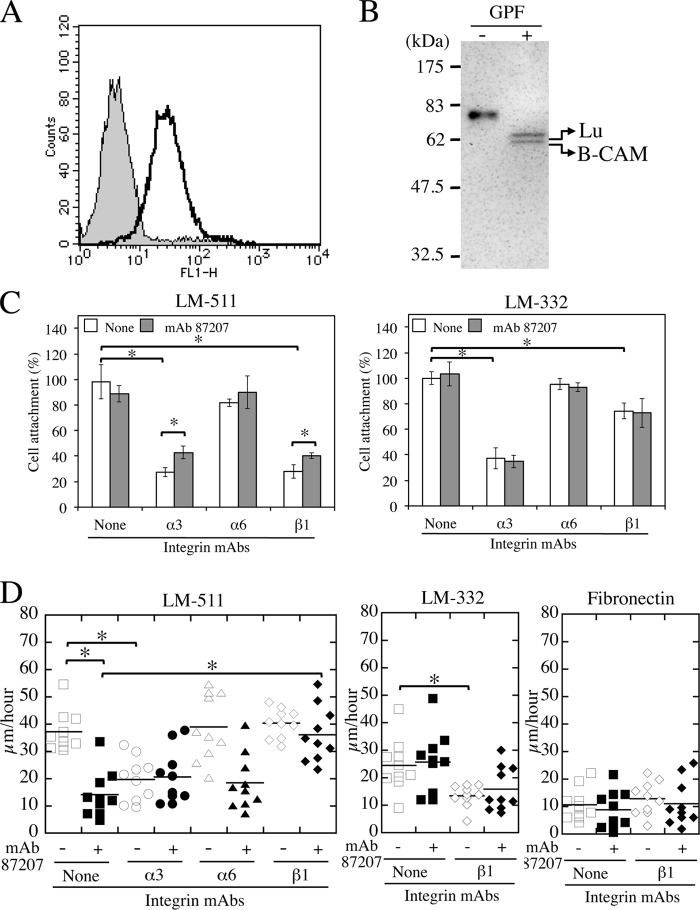
We also examined whether Lu/B-CAM is involved in tumor cell adhesion and migration using human lung adenocarcinoma A549 cells. Flow cytometric analysis showed that A549 cells expressed Lu/B-CAM on the cell surface (Fig. 6A). The monoclonal antibody to the extracellular domain of Lu/B-CAM used here cannot distinguish between the two variants. To determine which of the two isoforms was expressed in A549 cells, the cell lysates were analyzed by a combination of immunoprecipitation and immunoblotting (Fig. 6B). Proteins precipitated with a monoclonal antibody to the extracellular domain of Lu/B-CAM were treated with or without N-glycopeptidase F and then separated by SDS-PAGE for immunoblotting. The separated proteins were detected with a polyclonal antiserum to the extracellular domain of Lu/B-CAM. Two bands migrating at 60 and 64 kDa were detected, indicating that A549 cells express both Lu and B-CAM. As shown in Fig. 3, LM-511 is a potent cell-adhesive protein capable of being recognized by both α3β1 and α6β1 integrins as major surface receptors. Consistent with our previous studies, inhibition assays using function-blocking antibodies showed that the adhesion of A549 cells to LM-511 and LM-332 depended predominantly on integrin α3β1 and was inhibited significantly by anti-integrin α3 and β1 mAbs (Fig. 6C). The inhibitory effects of both antibodies on cell adhesion to LM-511 were also attenuated significantly in the presence of mAb 87207 to Lu/B-CAM. Meanwhile, mAb 87207 did not impact the inhibitory effects of both antibodies on cell adhesion to LM-332.
FIGURE 6.
Adhesion and migration of A549 lung adenocarcinoma cells expressing Lu/B-CAM on LM-511. A, expression levels of Lu/B-CAM (solid lines) in A549 cells. The gray area indicates the negative control. B, immunoblot analysis of Lu/B-CAM. A549 cell lysate was precipitated with anti Lu/B-CAM mAb (BRIC221). Precipitates were treated with (right lane) or without (left lane) glycopeptidase F (GPF), separated on a 10% gel under reducing conditions, and immunoblotted with an anti-Lu/B-CAM polyclonal antiserum. Lu and B-CAM migrated at 64 kDa and 60 kDa, respectively. Molecular mass standards are indicated. C, effects of mAb 87207 on adhesion of A549 cells to LM-511 and LM-332. A549 cells preincubated with mAb 87207 or/and function-blocking antibodies against the indicated integrin were added to LM-511 or LM-332-coated wells. After 30 min, the attached cells were stained and counted. Values are expressed as percentages of the number of cells adhering in the absence of antibody. Each column represents the mean of triplicate assays. Bars show S.D. *, p < 0.05 by Student's t test. D, migration of A549 cells on LM-511. A549 cells were plated on dishes coated with LM-511 (0.8 nm), LM-332 (0.8 nm) or fibronectin (40 nm). The cells were incubated with the indicated antibodies. Cell movements were tracked at 10-min intervals over a span of 8 h. Cell motility on LM-511 (left panel), LM-332 (center panel), and fibronectin (right panel) was evaluated by velocity (micrometers/hour) as described above. *, p < 0.01 by Dunnett's multiple comparison test.
Consistent with a previous report (13), the migration of A549 cells was significantly faster on LM-511 than on fibronectin (Fig. 6D). Although LM-332 also promoted cell migration, it was less effective than LM-511. The velocity of A549 cells on LM-511 was also comparable with the results in the previous report (13). However, the antibody against integrin α3 inhibited the migration of A549 cells on LM-511, but the integrin β1 antibody did not. We next examined whether Lu/B-CAM was involved in A549 cell migration on LM-511 using mAb 87207. As shown in Fig. 6D, the antibody significantly inhibited cell migration on LM-511. However, the inhibitory effects of mAb 87207 were reduced by the integrin β1 antibody. These results suggest that Lu/B-CAM and integrin α3β1 coordinately bind to laminin α5 and promote cell migration.
The Involvement of Integrins in Lu/B-CAM-mediated Cell Migration
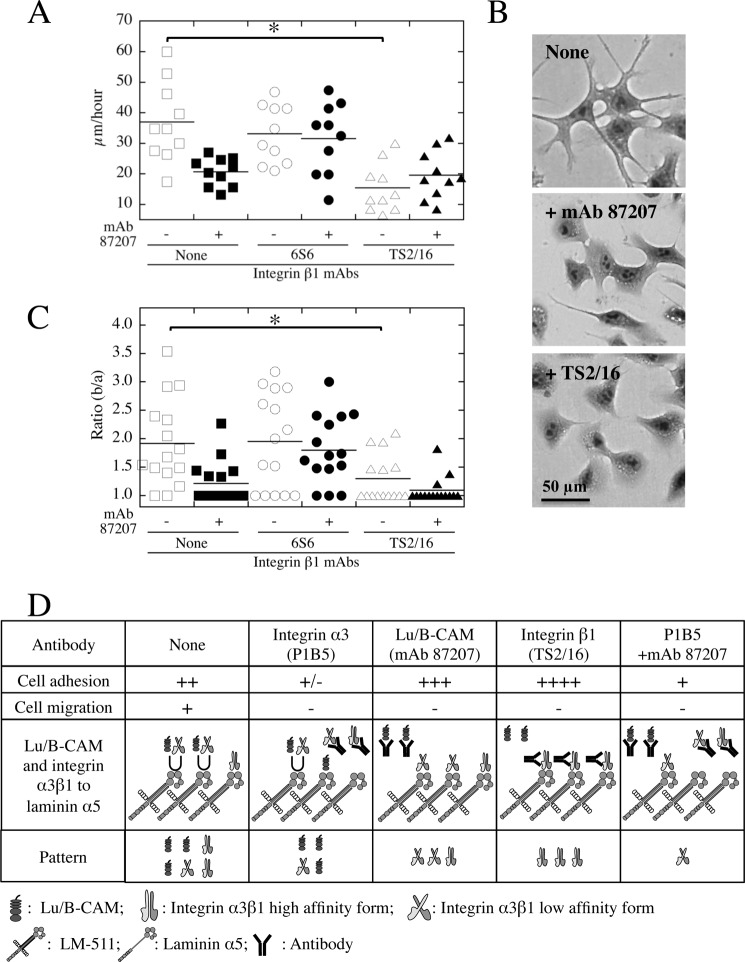
We also examined whether another function-blocking antibody against integrin β1 (6S6) inhibited cell migration on LM-511. However, as with the former antibody (mAb 13), 6S6 inhibited cell adhesion to LM-511 (data not shown) but not cell migration (Fig. 7A). The 6S6 antibody also abrogated the inhibitory effects of mAb 87207 on LM-511-mediated cell migration (Fig. 7A), indicating that Lu/B-CAM promotes cell migration indirectly. These results suggest that cell migration requires a reduction in the strength of integrin binding to laminin α5. Therefore, we hypothesized that the preferential binding of integrin α3β1 to laminin α5 causes a flattened cell shape with stable adhesion and impaired cell migration. To investigate this, we used an antibody against integrin β1 (TS2/16) that stimulates cell adhesion to extracellular matrix proteins (26). TS2/16-stimulated A549 cells exhibited a decreased cell migration velocity on LM-511 (Fig. 7A) and had a flat cell shape similar to mAb 87207-treated cells (B and C). These results indicate that weakened binding of integrin α3β1 to laminin α5 induced a spindle cell shape with pseudopods and promoted cell migration.
FIGURE 7.
Inhibitory effects of an activating integrin β1 mAb on cell migration and pseudopod elongation. A, migration of A549 cells on LM-511 in the presence of anti-integrin β1 (6S6 (inhibitory) and TS2/16 (activating)) and/or Lu/B-CAM (87207) mAbs. A549 cells were plated on dishes coated with LM-511 (0.8 nm). After cells adhered, the antibodies were added to the medium. Cell motility was evaluated by velocity (micrometers/hour) as described above. mAbs 87207 and TS2/16 slowed migration. B, morphology of A549 cells on LM-511. The cells were plated on dishes coated with LM-511 (0.8 nm). After cells adhered, they were incubated in the absence (top panel) or presence of mAb 87207 (center panel), and TS2/16 (bottom panel) mAbs. After another hour, the images of the stained cells were captured by charge-coupled device camera. C, quantification of cell morphology. The square area with (b) or without (a) pseudopods was measured as described above. Bars indicate means. *, p < 0.05 by Dunnett's multiple comparison test. Like mAb 87207, integrin-activating mAb TS2/16 inhibited not only pseudopod elongation but also migration on LM-511. D, summary of the adhesion and migration of A549 cells on LM-511 in the presence of the different antibodies. Cell adhesions are shown as follows: ++++, robust adhesion; +++, stable adhesion; ++, weakened stable adhesion; +, weak adhesion; ±, weak or no adhesion. Cell migrations are shown as follows: +, promoted velocity of cells more than that on fibronectin; −, comparable with velocity of cells on fibronectin. U, competitive binding of Lu/B-CAM and integrin α3β1 to laminin α5.
In Fig. 7D, we summarized the binding pattern of Lu/B-CAM and integrin α3β1 to laminin α5 in the presence of antibodies. The adhesion of A549 cells to the substrate is the first step leading to migration. Because integrin α3β1 is a primary receptor for adhesion of A549 cells to LM-511, the integrin α3 inhibitory mAb P1B5 also leads to cell detachment and inhibits cell migration. In addition, the stable cell adhesion in the presence of mAbs 87207 or TS2/16 also inhibits cell migration. These results suggest that weakened cell adhesion is required for A549 cell migration on LM-511. The negative effect of mAb P1B5 is partially cancelled in the presence of mAb 87207, indicating that Lu/B-CAM competitively inhibits the binding of integrin α3β1. On the basis of all of these results, we conclude that the preferential binding of Lu/B-CAM to laminin α5 leads to only modulate cell adhesion, thus allowing cell migration.
A Possible Compensation in Lu/B-CAM-deficient Mice
Because Lu/B-CAM is a specific receptor for laminin α5, any defects in mice lacking Lu/B-CAM could phenocopy those in Lama5-null mice, or, on the basis of the results presented here, might result from enhanced binding of integrin α3β1 to laminin α5. However, although mice lacking Lu/B-CAM exhibited minor defects in kidney and intestine, they were healthy and developed normally (31). We also produced mice lacking Lu/B-CAM (28) and examined the expression of integrin α3β1 in embryos. As shown in Fig. 8, in wild-type epidermis, integrin α3β1 was concentrated along with Lu/B-CAM at the basal cell surface adjacent to the epidermal basement membrane. In Lu/B-CAM knockout mice, integrin α3β1 exhibited a more basolateral localization. Therefore, the lack of Lu/B-CAM function may be compensated by a shift of integrin α3β1 away from the basal cell surface.
FIGURE 8.
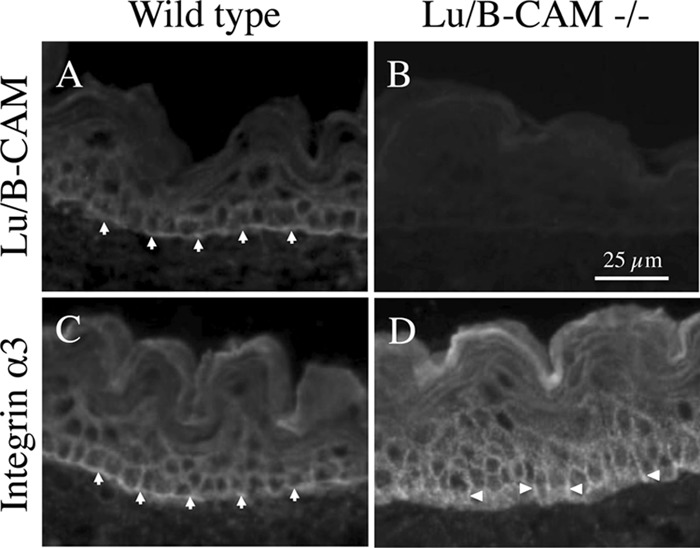
The localization of integrin α3 in Lu/B-CAM-deficient mice. Frozen sections of embryonic skin were stained with antibodies to Lu/B-CAM (A and B) and integrin α3 (C and D). Lu/B-CAM and integrin α3 were localized mainly to the basal cell surface in wild-type tissue (arrows). Integrin α3 was also observed at the basolateral region in Lu/B-CAM deficient cells (arrowheads).
DISCUSSION
In our previous study we showed that Lu/B-CAM and integrin α3β1 competitively bind to the LG domain of laminin α5 (Fig. 9A). On the basis of these results, we investigated the mechanisms of cell adhesion and migration on LM-511. Using an activating antibody to integrin β1, we determined that the high-affinity form of integrin α3β1 occupied the binding site on laminin α5 (Fig. 9B). In addition, Lu/B-CAM competed with a low-affinity form of integrin α3β1 for binding laminin α5. Overexpression of Lu/B-CAM in human fibrosarcoma cells led them to adopt a spindle cell shape with elongated pseudopods when plated on LM-511. On the other hand, control cells exhibited a flattened cell shape with lamellipodia. Consistent with these morphological distinctions, Lu/B-CAM suppressed cell adhesion to LM-511. It is unlikely that the binding of Lu/B-CAM to laminin α5 triggers an intercellular cascade to promote cell adhesion. Therefore, we conclude that the binding of Lu/B-CAM acts to disturb integrin-mediated cell attachment to laminin α5 rather than to promote cell anchoring.
FIGURE 9.
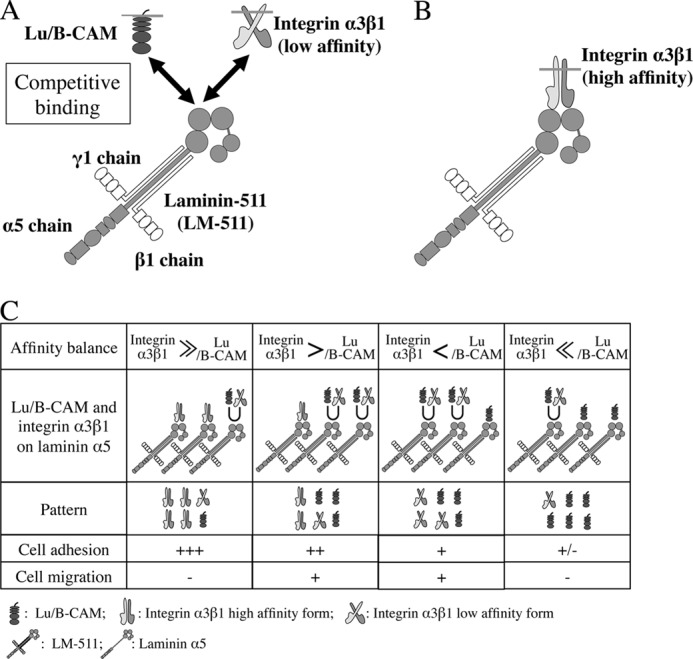
Schematic of the role of Lu/B-CAM in cell adhesion to and migration on LM-511. A, competitive binding between Lu/B-CAM and integrin α3β1 (low affinity) for laminin α5. Our previous study showed that the binding sites for Lu/B-CAM and integrin α3β1 are within the LG1–3 module of laminin α5 (26). B, preferential binding of integrin α3β1 (high affinity) to laminin α5. When integrin α3β1 occupies the binding site on laminin α5, Lu/B-CAM cannot bind to it. C, preferential binding of integrins to laminin α5 stimulates cascades to promote cell adhesion and maintains the non-migratory state with a flattered cell shape (left). The binding of Lu/B-CAM to laminin α5 leads to a cell morphology with pseudopods and promotes cell migration (center left and center right). Preferential binding of Lu/B-CAM to laminin α5 leads to weak cell adhesion or detachment (right). Cell adhesion is indicated as follows: +++, stable adhesion; ++, weakened stable adhesion; +, weak adhesion; ±, weak or no adhesion. Cell migration is indicated as follows: +, promoted cell migration; −, reduced cell migration. U, competitive binding of Lu/B-CAM and integrin α3β1 to laminin α5.
The attachment of control HT1080 cells to LM-511 was inhibited by the combination of anti-α3 and -α6 integrin antibodies or anti-β1 integrin antibody, as described in our previous study (16). However, here the inhibition was incomplete, perhaps because we used a trypsin-free detachment method that prevented integrin damage. On the other hand, the combination of anti-integrin α3 and α6 or β1 mAb(s) completely inhibited the attachment of Lu/B-CAM transfectants to LM-511. This result shows that the binding of Lu/B-CAM additionally inhibits cell adhesion to LM-511 in the presence of integrin antibodies. Therefore, because mAb 87207 blocked the additional inhibition of Lu/B-CAM binding, the cell adhesion to LM-511 was partially restored in the presence of integrin-blocking antibodies. This indicates that when Lu/B-CAM binds to laminin α5, it inhibits cell adhesion through integrins. In addition, the attachment of A549 cells to LM-511 was inhibited by mAbs to integrin α3 and β1 but not to α6, as described in our previous study (15). The inhibitory effect of anti-integrin α3 mAb was slightly impaired by mAb 87207. Overexpression of Lu/B-CAM led to weak attachment to LM-511 and a spindle cell shape. Because Lu/B-CAM and integrins compete for binding to laminin α5, we conclude that the binding of Lu/B-CAM to laminin α5 disturbs cell adhesion through integrins.
Gu et al. (13) showed that LM-511 promotes migration of A549 cells via integrin α3β1. As in that study, we found that migration of A549 cells on LM-511 was inhibited by anti-integrin α3 mAb. However, although anti-integrin β1 mAb inhibited cell adhesion to LM-511, the antibody had no inhibitory effects on cell migration. Because anti-integrin β1 mAb reacts with not only integrin α3β1 but also all integrins containing β1, the antibody may be less effective on cell migration through integrin α3β1. mAb 87207 also inhibited migration of A549 cells on LM-511, but it did not cancel the inhibitory effect of anti-integrin α3 mAb on the migration of A549 cells. This indicates that integrin α3β1, rather than Lu/B-CAM, plays a pivotal role in the migration of A549 cells on LM-511. Meanwhile an activating antibody against integrin β1, TS2/16, promoted cell adhesion and a flattened cell shape on LM-511 and suppressed cell migration, similar to mAb 87207. These results suggest that the binding of integrin α3β1 to laminin α5 suppresses cell migration, and we conclude that weakened binding of integrin α3β1 is required for active cell migration on LM-511. As summarized in Fig. 9C, Lu/B-CAM is a receptor that modulates the binding of integrin α3β1 to laminin α5 and indirectly promotes cell migration.
Lu and B-CAM promote cell migration on LM-511 at comparable levels. The Arg573Lys574 motif in the shared cytoplasmic tail of Lu and B-CAM attaches to the spectrin cytoskeleton and regulates cell adhesive activity (32, 33). A recent study showed that a Lu/B-CAM-αII spectrin interaction impacts actin organization in epithelial cells (34). This motif in Lu/B-CAM may be involved in intracellular signaling that mediates cell migration on LM-511. Because the cytoplasmic tail of Lu carries an SH3 binding motif, a dileucine motif, and potential phosphorylation sites, Lu has been investigated as a signaling molecule (21). Protein kinase A phosphorylates Ser621 in the cytoplasmic tail and stimulates adhesion of sickled red blood cells to laminin under flow conditions (35). A constitutively active JAK2 promotes Lu-mediated red cell adhesion through the Rap1/Akt pathway (36). The abnormal adhesion of red blood cells to laminin α5 is due to the Ser621 phosphorylation of Lu/BCAM by the JAK2/Rap1/Akt pathway. These motifs may regulate cell migration on LM-511 through the inside-out signaling induced by mediators.
In Lu/B-CAM-deficient mice, the lack of Lu/B-CAM function seems to be compensated by a shift of integrin α3β1 from a primarily basal location to the basolateral region. Of relevance to this, a dileucine motif at 608–609 in the cytoplasmic tail of Lu mediates basolateral sorting of Lu in epithelial cells (37). Thus, in addition to the competitive binding of Lu/B-CAM and integrins to laminin α5, the basolateral sorting of the receptors is a possible mechanism for modulating cell-matrix interactions.
In multiple steps of invasion and metastasis, tumor cells must adhere to and migrate through basement membranes. Laminin α5 is a major component of basement membranes and stably anchors normal epithelial cells in vivo. In contrast, several studies have shown that laminin α5 promotes not only cell adhesion but also tumor cell migration. Thus, laminin α5 can be viewed as a double-edged molecule in normal and tumor cells. This is because tumor cells that arise from sequential genetic mutations could have an altered pattern of cell surface molecule expression, including laminin receptors. In this study we showed that the preferential binding of Lu/B-CAM to laminin α5 plays a crucial role in tumor cell migration. Therefore, in the future it will be necessary to clarify the mechanisms modulating expression of Lu/B-CAM in tumor cells. We also showed that a function-blocking antibody against Lu/B-CAM effectively inhibits cell migration of lung carcinoma cells on LM-511. The antibody could be useful for developing biological drugs to inhibit tumor invasion and metastasis.
Acknowledgments
We thank Takayuki Hamakubo, Masanari Tange, and Yurina Kashiwaya for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK078314 (to J. H. M.). This work was also supported by Ministry of Education, Sciences Sports and Culture, Japan grants (to Y. K.).

This article contains supplemental Movies 1 and 2 and legends.
- Lu
- Lutheran
- B-CAM
- basal cell adhesion molecule
- Sol-Lu
- soluble Lutheran
- CDB
- cell dissociation buffer
- Lu-Fc
- Fc-tagged Lu/B-CAM extracellular domain.
REFERENCES
- 1. Kleinman H. K., Martin G. R. (2005) Matrigel. Basement membrane matrix with biological activity. Semin. Cancer Biol. 15, 378–386 [DOI] [PubMed] [Google Scholar]
- 2. Aumailley M., Bruckner-Tuderman L., Carter W. G., Deutzmann R., Edgar D., Ekblom P., Engel J., Engvall E., Hohenester E., Jones J. C., Kleinman H. K., Marinkovich M. P., Martin G. R., Mayer U., Meneguzzi G., Miner J. H., Miyazaki K., Patarroyo M., Paulsson M., Quaranta V., Sanes J. R., Sasaki T., Sekiguchi K., Sorokin L. M., Talts J. F., Tryggvason K., Uitto J., Virtanen I., von der Mark K., Wewer U. M., Yamada Y., Yurchenco P. D. (2005) A simplified laminin nomenclature. Matrix Biol. 24, 326–332 [DOI] [PubMed] [Google Scholar]
- 3. Durbeej M. (2010) Laminins. Cell Tissue Res. 339, 259–268 [DOI] [PubMed] [Google Scholar]
- 4. Miner J. H., Patton B. L., Lentz S. I., Gilbert D. J., Snider W. D., Jenkins N. A., Copeland N. G., Sanes J. R. (1997) The laminin α chains. Expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J. Cell Biol. 137, 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorokin L. M., Pausch F., Frieser M., Kröger S., Ohage E., Deutzmann R. (1997) Developmental regulation of the laminin α5 chain suggests a role in epithelial and endothelial cell maturation. Dev. Biol. 189, 285–300 [DOI] [PubMed] [Google Scholar]
- 6. Tanimizu N., Kikkawa Y., Mitaka T., Miyajima A. (2012) α1- and α5-containing laminins regulate the development of bile ducts via β1 integrin signals. J. Biol. Chem. 287, 28586–28597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spenlé C., Simon-Assmann P., Orend G., Miner J. H. (2013) Laminin α5 guides tissue patterning and organogenesis. Cell Adh. Migr. 7, 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shannon M. B., Patton B. L., Harvey S. J., Miner J. H. (2006) A hypomorphic mutation in the mouse laminin α5 gene causes polycystic kidney disease. J. Am. Soc. Nephrol. 17, 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pouliot N., Kusuma N. (2013) Laminin-511. A multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis. Cell Adh. Migr. 7, 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kikkawa Y., Sudo R., Kon J., Mizuguchi T., Nomizu M., Hirata K., Mitaka T. (2008) Laminin α 5 mediates ectopic adhesion of hepatocellular carcinoma through integrins and/or Lutheran/basal cell adhesion molecule. Exp. Cell Res. 314, 2579–2590 [DOI] [PubMed] [Google Scholar]
- 11. Gao J., DeRouen M. C., Chen C. H., Nguyen M., Nguyen N. T., Ido H., Harada K., Sekiguchi K., Morgan B. A., Miner J. H., Oro A. E., Marinkovich M. P. (2008) Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 22, 2111–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritié L., Spenlé C., Lacroute J., Bolcato-Bellemin A. L., Lefebvre O., Bole-Feysot C., Jost B., Klein A., Arnold C., Kedinger M., Bagnard D., Orend G., Simon-Assmann P. (2012) Abnormal Wnt and PI3Kinase signaling in the malformed intestine of lama5-deficient mice. PloS ONE 7, e37710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu J., Sumida Y., Sanzen N., Sekiguchi K. (2001) Laminin-10/11 and fibronectin differentially regulate integrin-dependent Rho and Rac activation via p130(Cas)-CrkII-DOCK180 pathway. J. Biol. Chem. 276, 27090–27097 [DOI] [PubMed] [Google Scholar]
- 14. Gu J., Fujibayashi A., Yamada K. M., Sekiguchi K. (2002) Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways. J. Biol. Chem. 277, 19922–19928 [DOI] [PubMed] [Google Scholar]
- 15. Kikkawa Y., Sanzen N., Sekiguchi K. (1998) Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. Laminin-10/11 mediates cell adhesion through integrin α3 β1. J. Biol. Chem. 273, 15854–15859 [DOI] [PubMed] [Google Scholar]
- 16. Kikkawa Y., Sanzen N., Fujiwara H., Sonnenberg A., Sekiguchi K. (2000) Integrin binding specificity of laminin-10/11. Laminin-10/11 are recognized by α 3 β 1, α 6 β 1 and α 6 β 4 integrins. J. Cell Sci. 113, 869–876 [DOI] [PubMed] [Google Scholar]
- 17. Shimizu H., Hosokawa H., Ninomiya H., Miner J. H., Masaki T. (1999) Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J. Biol. Chem. 274, 11995–12000 [DOI] [PubMed] [Google Scholar]
- 18. El Nemer W., Gane P., Colin Y., Bony V., Rahuel C., Galactéros F., Cartron J. P., Le Van Kim C. (1998) The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem. 273, 16686–16693 [DOI] [PubMed] [Google Scholar]
- 19. Udani M., Zen Q., Cottman M., Leonard N., Jefferson S., Daymont C., Truskey G., Telen M. J. (1998) Basal cell adhesion molecule/Lutheran protein. The receptor critical for sickle cell adhesion to laminin. J. Clin. Invest. 101, 2550–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kikkawa Y., Moulson C. L., Virtanen I., Miner J. H. (2002) Identification of the binding site for the Lutheran blood group glycoprotein on laminin α 5 through expression of chimeric laminin chains in vivo. J. Biol. Chem. 277, 44864–44869 [DOI] [PubMed] [Google Scholar]
- 21. Parsons S. F., Mallinson G., Holmes C. H., Houlihan J. M., Simpson K. L., Mawby W. J., Spurr N. K., Warne D., Barclay A. N., Anstee D. J. (1995) The Lutheran blood group glycoprotein, another member of the immunoglobulin superfamily, is widely expressed in human tissues and is developmentally regulated in human liver. Proc. Natl. Acad. Sci. U.S.A. 92, 5496–5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mankelow T. J., Burton N., Stefansdottir F. O., Spring F. A., Parsons S. F., Pedersen J. S., Oliveira C. L., Lammie D., Wess T., Mohandas N., Chasis J. A., Brady R. L., Anstee D. J. (2007) The Laminin 511/521-binding site on the Lutheran blood group glycoprotein is located at the flexible junction of Ig domains 2 and 3. Blood 110, 3398–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burton N. M., Brady R. L. (2008) Molecular structure of the extracellular region of Lutheran blood group glycoprotein and location of the laminin binding site. Blood Cells Mol. Dis. 40, 446–448 [DOI] [PubMed] [Google Scholar]
- 24. Campbell I. G., Foulkes W. D., Senger G., Trowsdale J., Garin-Chesa P., Rettig W. J. (1994) Molecular cloning of the B-CAM cell surface glycoprotein of epithelial cancers. A novel member of the immunoglobulin superfamily. Cancer Res. 54, 5761–5765 [PubMed] [Google Scholar]
- 25. Moulson C. L., Li C., Miner J. H. (2001) Localization of Lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin α5 in vivo. Dev. Dyn. 222, 101–114 [DOI] [PubMed] [Google Scholar]
- 26. Kikkawa Y., Sasaki T., Nguyen M. T., Nomizu M., Mitaka T., Miner J. H. (2007) The LG1–3 tandem of laminin α5 harbors the binding sites of Lutheran/basal cell adhesion molecule and α3β1/α6β1 integrins. J. Biol. Chem. 282, 14853–14860 [DOI] [PubMed] [Google Scholar]
- 27. Kikkawa Y., Miwa T., Tohara Y., Hamakubo T., Nomizu M. (2011) An antibody to the Lutheran glycoprotein (Lu) recognizing the LU4 blood type variant inhibits cell adhesion to laminin α5. PloS ONE 6, e23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishimune H., Valdez G., Jarad G., Moulson C. L., Müller U., Miner J. H., Sanes J. R. (2008) Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J. Cell Biol. 182, 1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richard M., Perreault J., Gane P., el Nemer W., Cartron J. P., St-Louis M. (2006) Phage-derived monoclonal anti-Lu. Transfusion 46, 1011–1017 [DOI] [PubMed] [Google Scholar]
- 30. Alais S., Allioli N., Pujades C., Duband J. L., Vainio O., Imhof B. A., Dunon D. (2001) HEMCAM/CD146 downregulates cell surface expression of β1 integrins. J. Cell Sci. 114, 1847–1859 [DOI] [PubMed] [Google Scholar]
- 31. Rahuel C., Filipe A., Ritie L., El Nemer W., Patey-Mariaud N., Eladari D., Cartron J. P., Simon-Assmann P., Le Van Kim C., Colin Y. (2008) Genetic inactivation of the laminin α5 chain receptor Lu/BCAM leads to kidney and intestinal abnormalities in the mouse. Am. J. Physiol. Renal Physiol. 294, F393–F406 [DOI] [PubMed] [Google Scholar]
- 32. Kroviarski Y., El Nemer W., Gane P., Rahuel C., Gauthier E., Lecomte M. C., Cartron J. P., Colin Y., Le Van Kim C. (2004) Direct interaction between the Lu/B-CAM adhesion glycoproteins and erythroid spectrin. Br. J. Haematol. 126, 255–264 [DOI] [PubMed] [Google Scholar]
- 33. An X., Gauthier E., Zhang X., Guo X., Anstee D. J., Mohandas N., Chasis J. A. (2008) Adhesive activity of Lu glycoproteins is regulated by interaction with spectrin. Blood 112, 5212–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collec E., Lecomte M. C., El Nemer W., Colin Y., Le Van Kim C. (2011) Novel role for the Lu/BCAM-spectrin interaction in actin cytoskeleton reorganization. Biochem. J. 436, 699–708 [DOI] [PubMed] [Google Scholar]
- 35. Gauthier E., Rahuel C., Wautier M. P., El Nemer W., Gane P., Wautier J. L., Cartron J. P., Colin Y., Le Van Kim C. (2005) Protein kinase A-dependent phosphorylation of Lutheran/basal cell adhesion molecule glycoprotein regulates cell adhesion to laminin α5. J. Biol. Chem. 280, 30055–30062 [DOI] [PubMed] [Google Scholar]
- 36. De Grandis M., Cambot M., Wautier M. P., Cassinat B., Chomienne C., Colin Y., Wautier J. L., Le Van Kim C., El Nemer W. (2013) JAK2V617F activates Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood 121, 658–665 [DOI] [PubMed] [Google Scholar]
- 37. El Nemer W., Colin Y., Bauvy C., Codogno P., Fraser R. H., Cartron J. P., Le Van Kim C. L. (1999) Isoforms of the Lutheran/basal cell adhesion molecule glycoprotein are differentially delivered in polarized epithelial cells. Mapping of the basolateral sorting signal to a cytoplasmic di-leucine motif. J. Biol. Chem. 274, 31903–31908 [DOI] [PubMed] [Google Scholar]
- 38. O'Gorman S., Fox D. T., Wahl G. M. (1991) Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science 251, 1351–1355 [DOI] [PubMed] [Google Scholar]