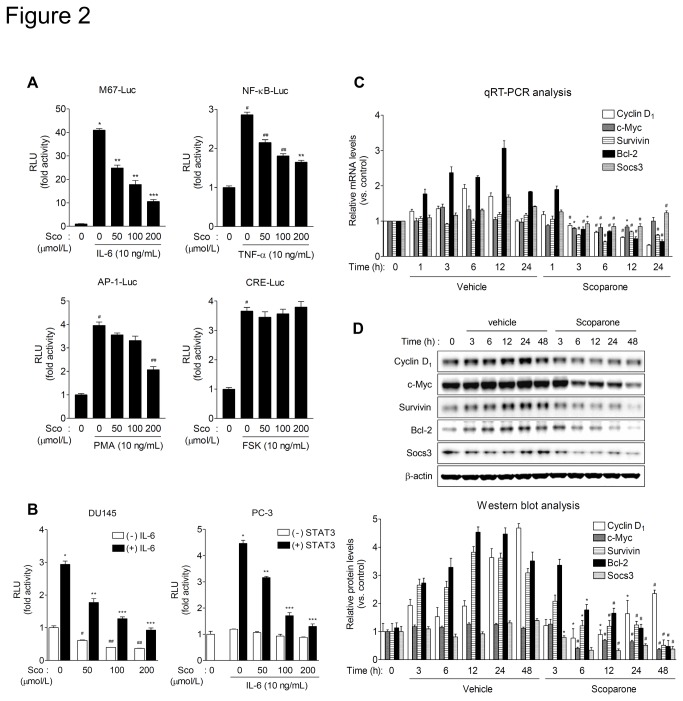
Figure 2. Scoparone inhibits constitutive and IL-6-stimulated transcriptional activity of STAT3.
A. The effect of scoparone on the activities of transcriptional factors. HepG2 cells were transiently cotransfected with various luciferase reporter plasmids driven by synthetic promoters containing binding sites for relevant transcription factors. At 24 h after transfection, cells were treated with the appropriate upstream stimulators and scoparone for 24 h, and then harvested for luciferase and β-galactosidase assays. RLU, relative luminescence units. Data are the means ± S.E.M. of three independent experiments, each performed in duplicate. * P < 0.0005, # P < 0.005 vs. reporter alone; ** P < 0.005, *** P < 0.001, ## P < 0.01 vs. stimulation. B. Scoparone inhibits both constitutive and IL-6-stimulated transcriptional activity of STAT3. DU145 cells were transiently transfected with M67-Luc, a synthetic promoter containing four copies of the STAT3 binding site. PC-3 cells were co-transfected with M67-Luc construct and the expression vector for wild-type STAT3. At 24 h after transfection, cells were treated with IL-6 in the presence of scoparone for 24 h. Data are the means ± S.E.M. of three independent experiments, each performed in duplicate. * P < 0.005, # P < 0.05, ## P < 0.01 vs. reporter alone; ** P < 0.01, *** P < 0.005 vs. IL-6 stimulation. C and D. qRT-PCR and Western blot analyses of STAT3 downstream target genes. DU145 cells were treated with scoparone (0.5 mmol/L) for the indicated times, and then harvested for qRT-PCR (C) and Western blot (D) analyses. mRNA levels of STAT3 target genes were determined by qRT-PCR analysis (C) and normalized against the level of RPLP0/36B4. Data are expressed as means ± S.E.M. of three independent experiments, each performed in triplicate. * P < 0.05, # P < 0.005 vs. vehicle control at each time point. Protein expression levels (D) were quantified by densitometric analysis and normalized against the corresponding levels of β-actin. Data are expressed as means ± S.E.M. of three independent experiments. * P < 0.05, # P < 0.005 vs. vehicle control at each time point.