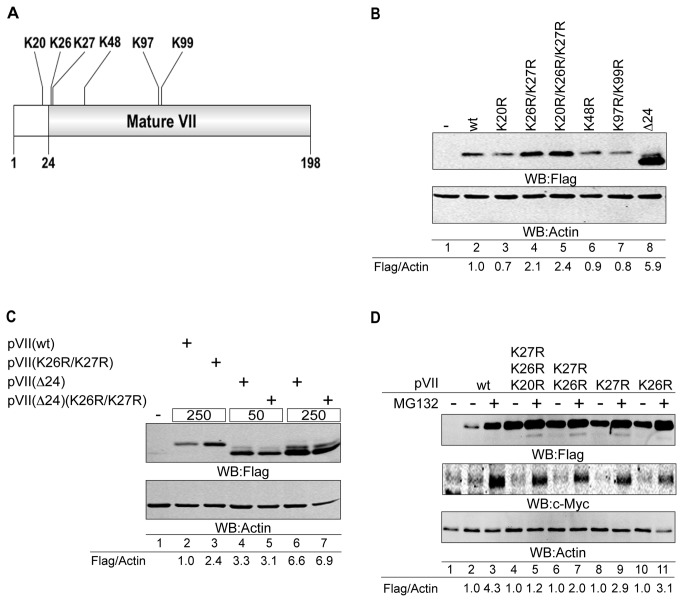
Figure 3. Lysines K26 and K27 control the Ad5 precursor pVII protein stability.
(A) Schematic localization of Ad5 pVII lysine (K) residues in the protein. (B) Mutation of lysines K26 and K27 increase the pVII protein stability. The expression of Flag-tagged pVII lysine mutant proteins was detected in transiently transfected HeLa cells. The relative expression levels of the Flag-tagged pVII proteins are shown as the fluorescence signal ratio of Flag/Actin proteins. Hyphen (-) indicates non-transfected cells. (C) K26/K27 mutation in the pVII(Δ24) protein does not increase the protein stability. Indicated proteins were transiently expressed in HeLa cells and their accumulation was analysed by Western blotting. Two different plasmid concentrations, 50ng (50) and 250ng (250), were used to express the pVII(Δ24)Flag proteins. Quantification of the Flag-tagged pVII proteins is shown as the fluorescence signal ratio of Flag/Actin proteins. Hyphen (-) indicates non-transfected cells. (D) Accumulation of the pVII mutant proteins in the presence of MG132. HEK293T cells transiently transfected with the pVII mutant proteins expressing plasmids were treated with 5μM MG132 for 12 hours. The quantification of Flag-tagged pVII proteins, shown below the image, is mean of two independent experiments. MG132-treated signals were related to DMSO-treated signals (-), which were set as 1. In all samples the Flag signals were normalized to the Actin signal (Flag/Actin). Anti-c-Myc antibody was used to monitor the MG132 treatment.